Chemicals list & Research Gallery
CAS number: 37294-28-3
Xyloglucan is a type of hemicellulose, a major component of plant cell walls, particularly in the primary cell wall. It's a polysaccharide made up of a backbone of (1->4)-linked β-D-glucan, with side chains of xylose, galactose, and sometimes fucose, attached to the backbone. Xyloglucan plays a crucial role in maintaining cell wall structure and is believed to be a key component in the network of cellulose and other polysaccharides that provides strength to plant cells.
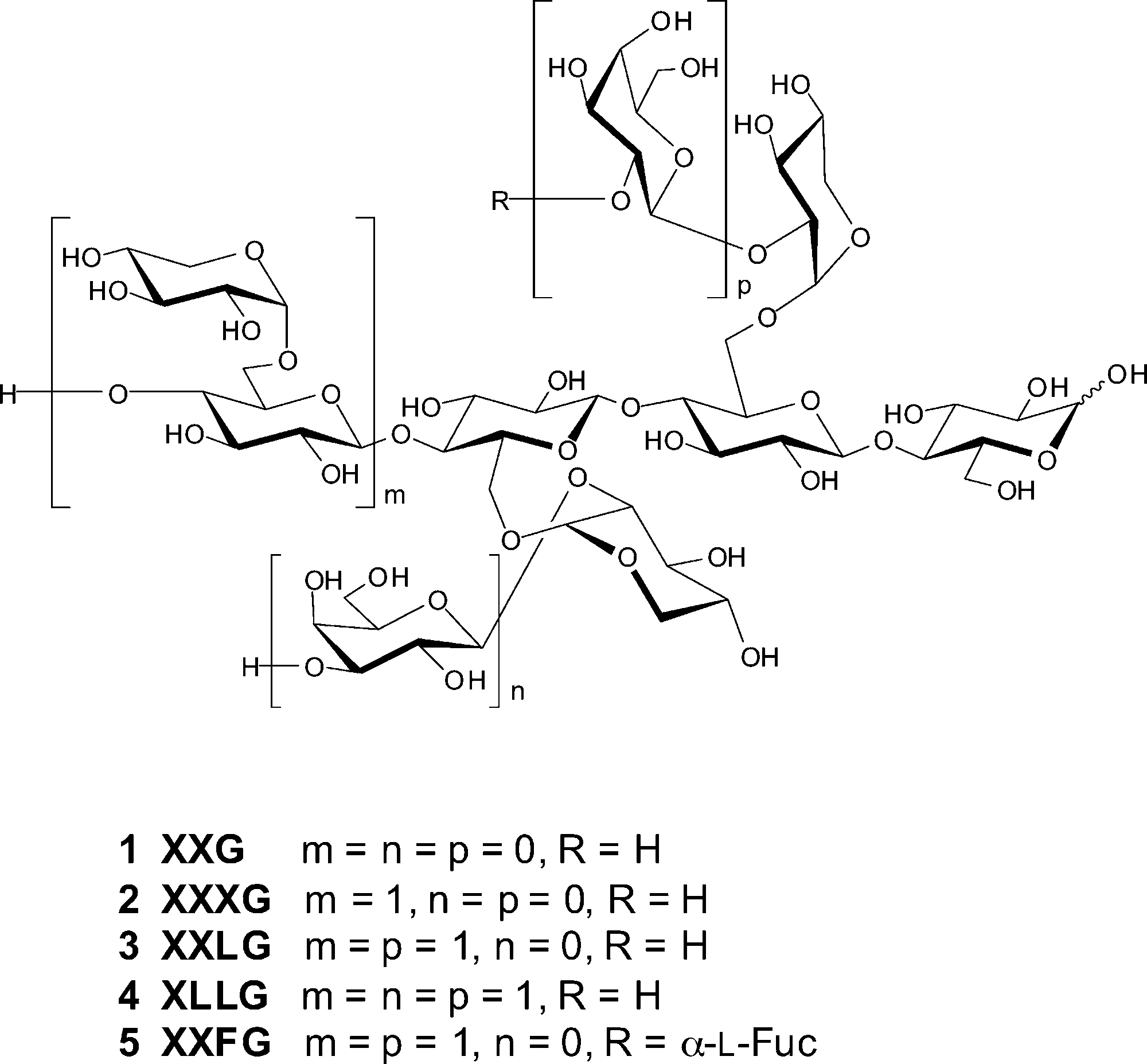
Structure of some common xyloglucan derivatives (XGOs) 1-5.
CAS number: 37294-30-7
Gratisin is a large, complex cyclic depsipeptide belongs to the class of synthetic macrocyclic peptidomimetics, engineered to mimic the structural and functional properties of natural peptides, often with improved stability and specificity. Gratisin features multiple chiral centers and functional groups, including amino, hydroxyl, isobutyl, isopropyl, and benzyl side chains, arranged in a highly stereospecific three-dimensional scaffold.
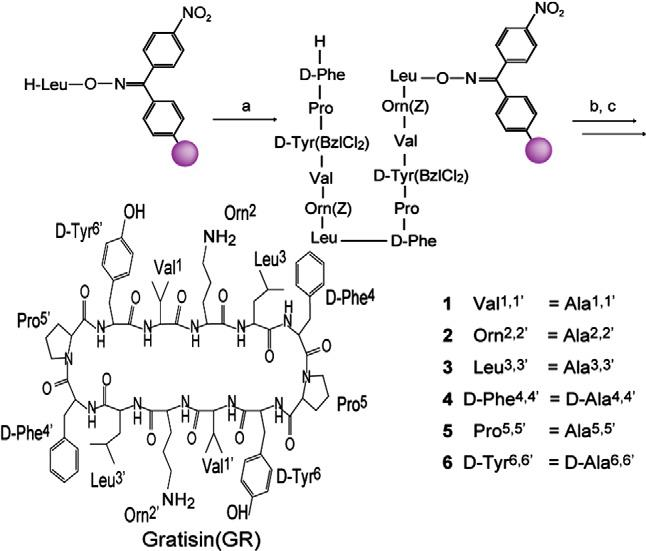
Syntheses of Gratisin and its Ala-substituted analogues 1–6.
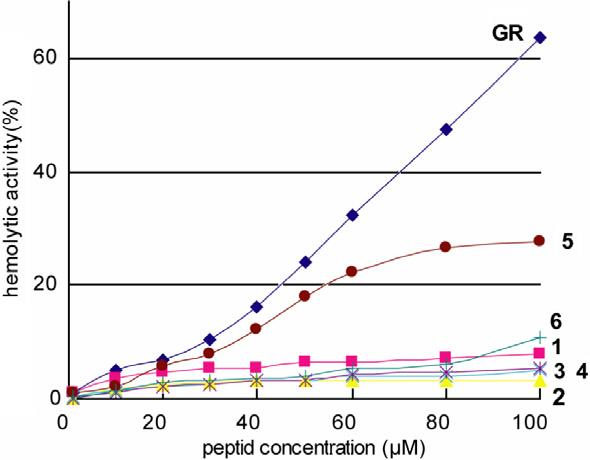
Dose dependence curves of hemolysis (%) induced by Gratisin and its Ala-substituted analogues 1–6.
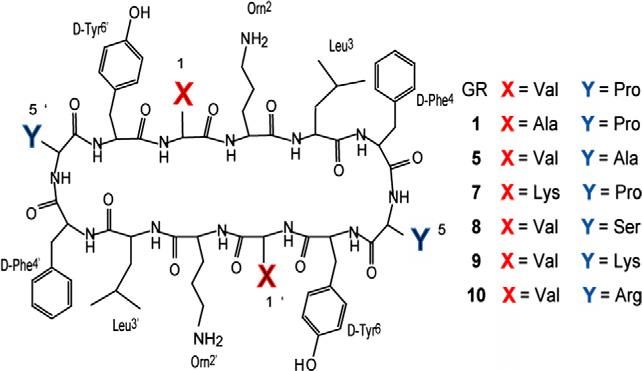
Primary structures of Gratisin and its analogues (1, 5, and 7–10)
CAS number: 37306-44-8
Triazole is a five-membered unsaturated aromatic heterocycle with more than two heteroatoms including two carbons and three nitrogens.
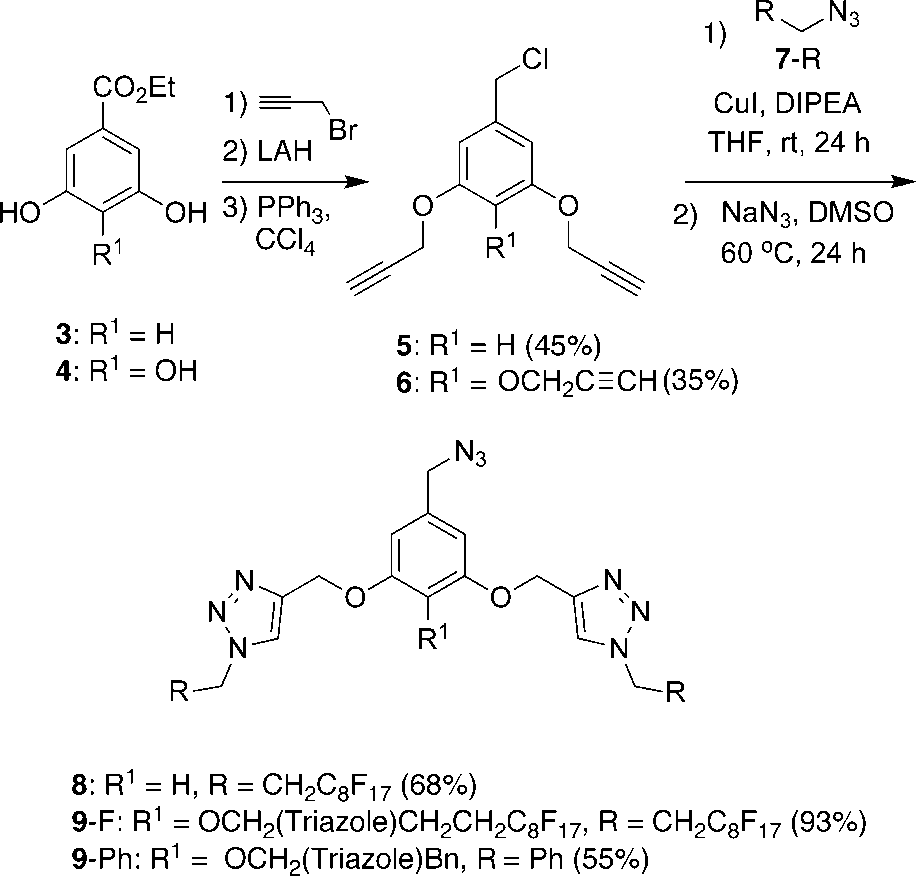
Synthesis of benzyl azides containing multiple triazole and perfluoroalkyl moieties
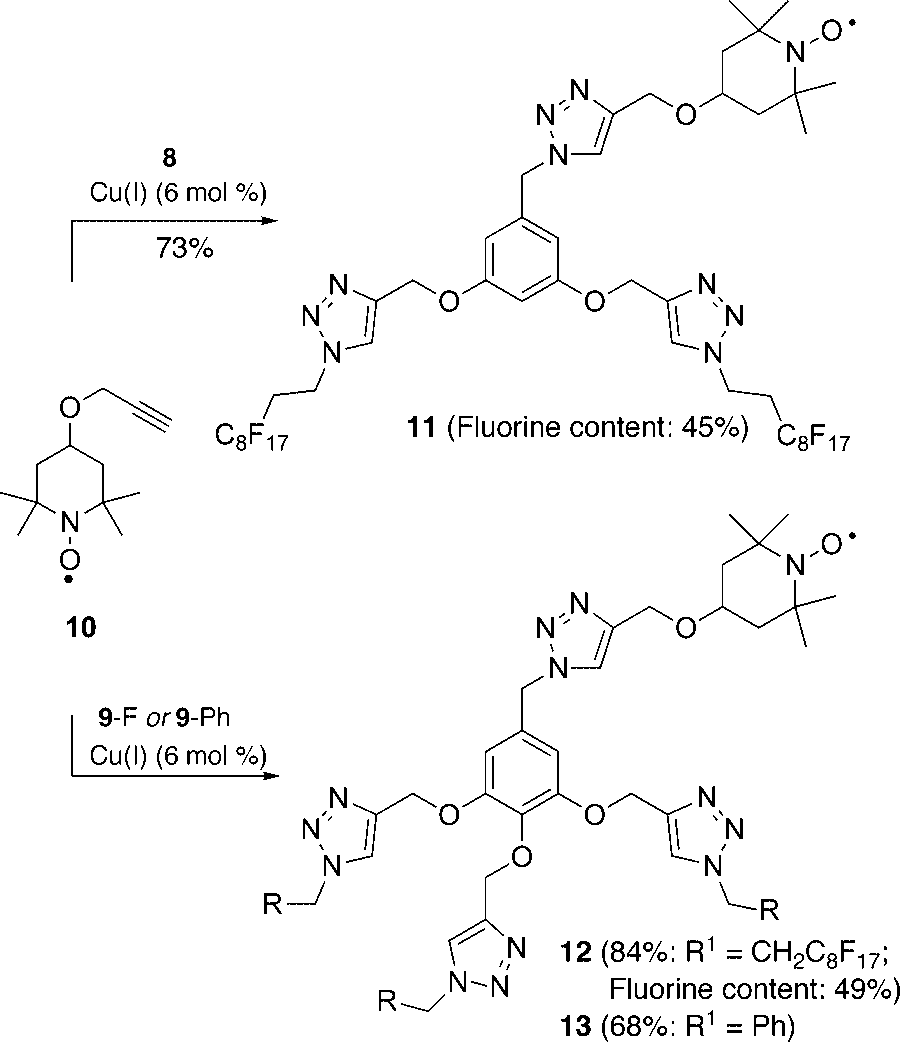
Synthesis of novel TEMPO reagents containing multiple triazole and perfluoroalkyl moieties
CAS number: 37497-65-7
2-piperideine is a piperideine.
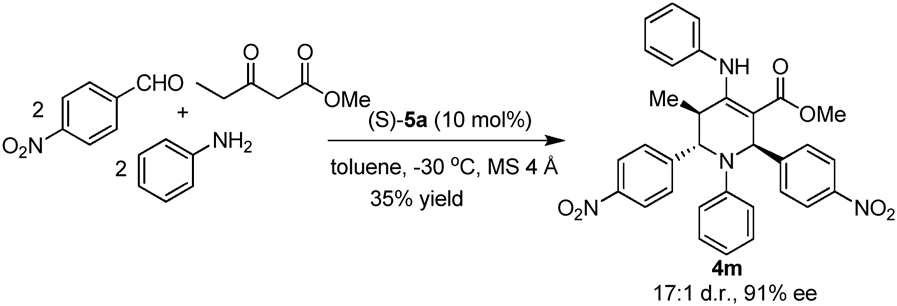
Synthesis of tetrahydropyridine 4m.
CAS number: 376348-65-1
Maraviroc is a CCR5 Co-receptor Antagonist. The mechanism of action of maraviroc is as a Chemokine Co-receptor 5 Antagonist.
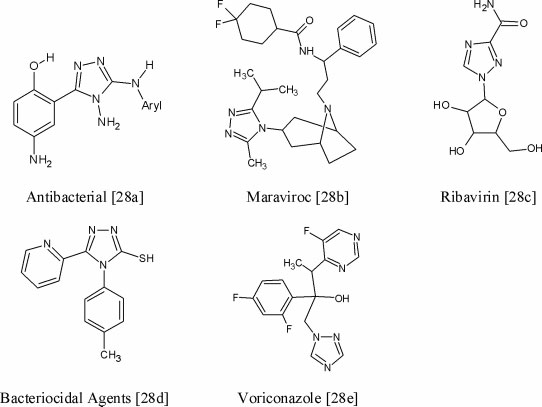
Examples of some bioactive compounds containing 1,2,4-triazole moiety.
CAS number: 37758-47-7
Ganglioside GM1 (ammonium salt), derived from bovine brain, is a type of ganglioside used in research, particularly for neurological studies. It's a monosialylated ganglioside, meaning it contains one sialic acid residue, and is known for its role as a functional receptor for Cholera toxin. Ganglioside GM1 is found in various cell types, including neurons, and is enriched in lipid rafts of cell membranes.
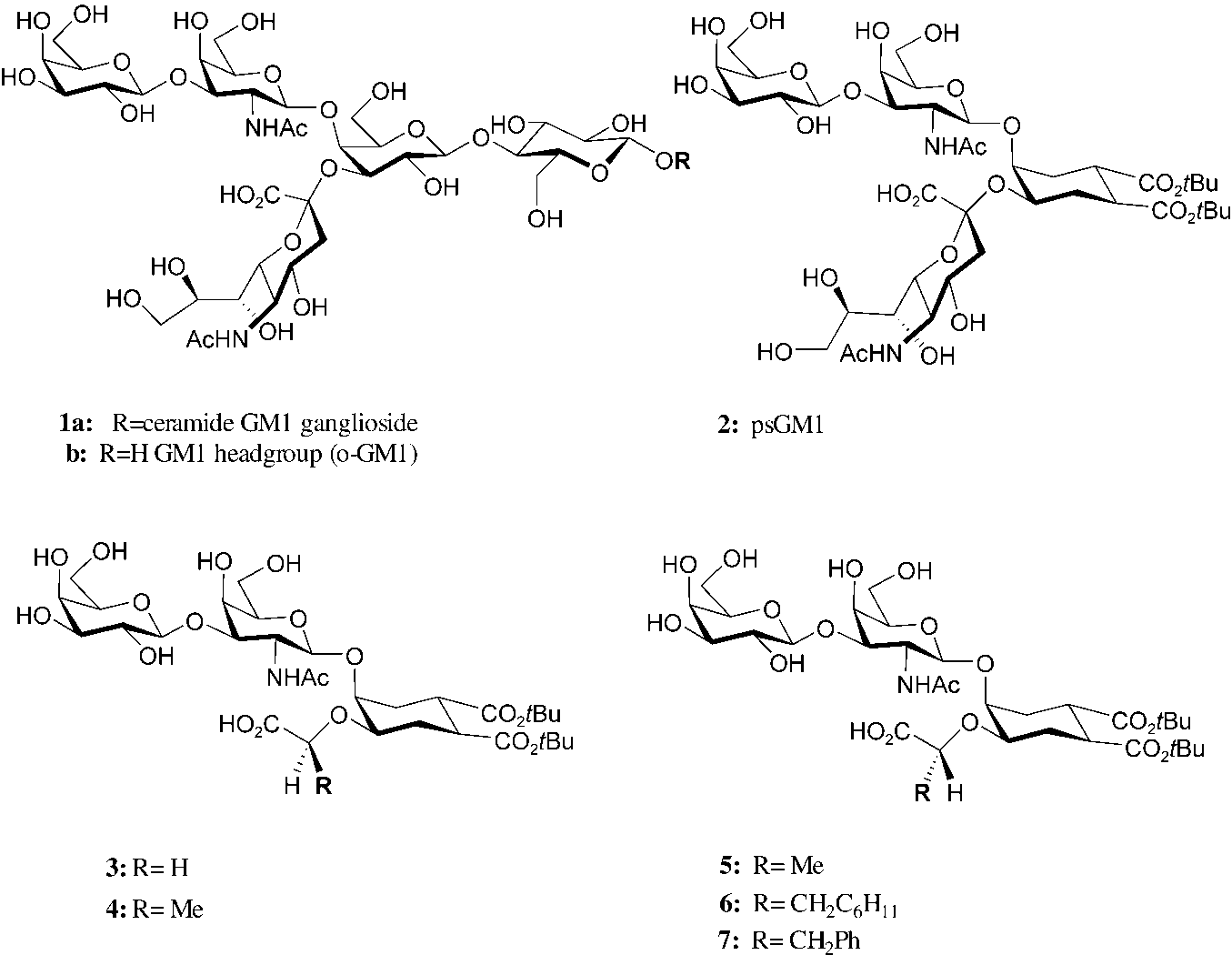
Ganglioside Gm1 and its mimics.
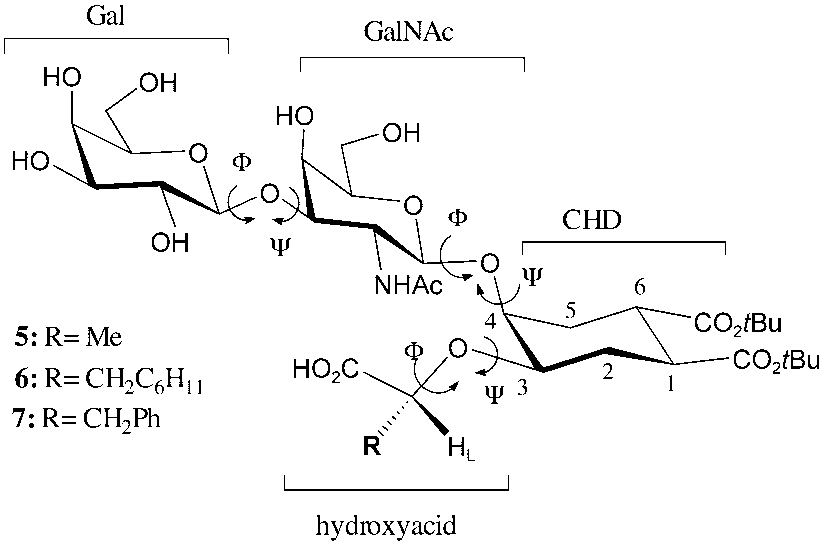
Gm1 mimics nomenclature, abbreviations, and definitions. Gal=galactose, GalNAc=N-acetyl galactosamine, CHD=cyclohexanediol, HL =H-L in the text and other figures.
CAS number: 378750-35-7
1,5-dihydro-4H-imidazol-4-one is an imidazolinone.
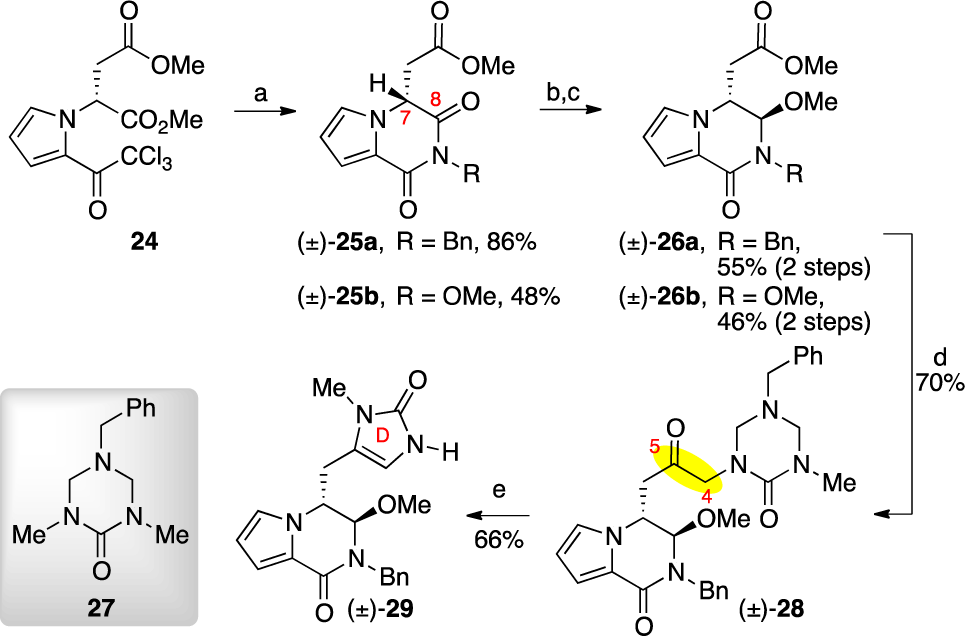
Synthesis of Imidazolone ( )-29
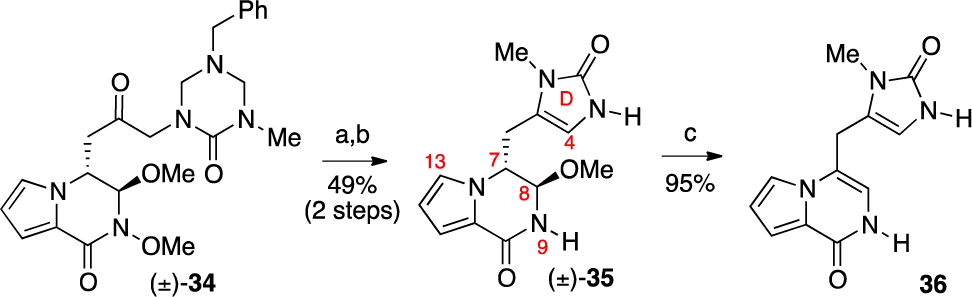
Synthesis of Imidazolone Derivative ( )-35 and Its Reactivity under Acidic Conditions
CAS number: 379270-37-8
Tenofovir alafenamide is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of chronic hepatitis B virus infection (HBV) in adults and children 6 years of age and older who weigh at least 55 lb (25 kg) and who meet certain requirements, as determined by a health care provider.
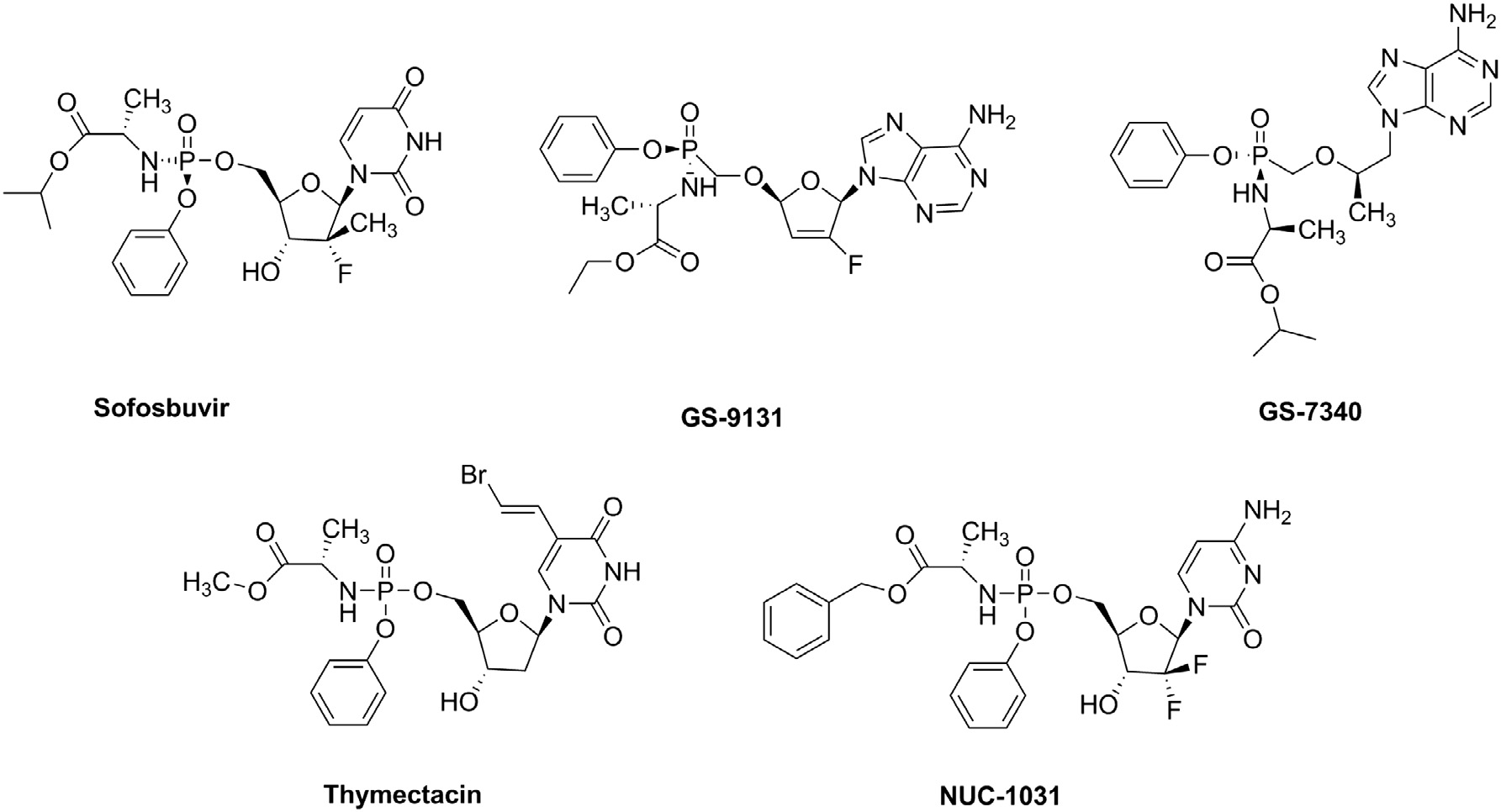
Known ProTides: SOFOSBUVIR, GS-9131, GS-7340, Thymectacin, NUC-1031.
CAS number: 37943-90-1
2-Pyridyldiphenylphosphine, also known as Diphenyl-2-pyridylphosphine, is a tertiary phosphine ligand featuring a phosphorus atom bonded to two phenyl groups and one 2-pyridyl group. This hybrid structure combines both soft (phosphorus) and hard (nitrogen) donor atoms, making it a versatile chelating ligand in coordination chemistry and homogeneous catalysis. Its ability to bridge metal centers or coordinate through both phosphorus and nitrogen allows it to stabilize a range of metal complexes, particularly in catalytic systems such as hydrogenation, hydroformylation, and cross-coupling reactions. The electron-donating properties and steric bulk of the phenyl groups, combined with the coordination flexibility of the pyridyl ring, contribute to its popularity in organometallic and inorganic chemistry.
 type, where N,O is diphenyl(2-pyridyl)phosphine oxide (L1), diphenyl(8-quinolyl)phosphine oxide (L2) or diphenyl(2-pyridylmethyl)phosphine oxide (L3), and P,P is DPEphos or a pair of PPh3 ligands. The complex structures are shown in Scheme 1.](http://www.wlxkc.cn/picture/5280276_02.png)
We synthesized six mixed-ligand CuI complexes of the [Cu(N,O)(P,P)](BF4) type, where N,O is diphenyl(2-pyridyl)phosphine oxide (L1), diphenyl(8-quinolyl)phosphine oxide (L2) or diphenyl(2-pyridylmethyl)phosphine oxide (L3), and P,P is DPEphos or a pair of PPh3 ligands. The complex structures are shown in Scheme 1.
CAS number: 380899-24-1
SB-649868 is under investigation in clinical trial NCT01030939 (Study to Investigate Safety, Tolerability, Pharmacokinetics and Cardiac Function After Repeat Doses of SB-649868 in Healthy-volunteers).
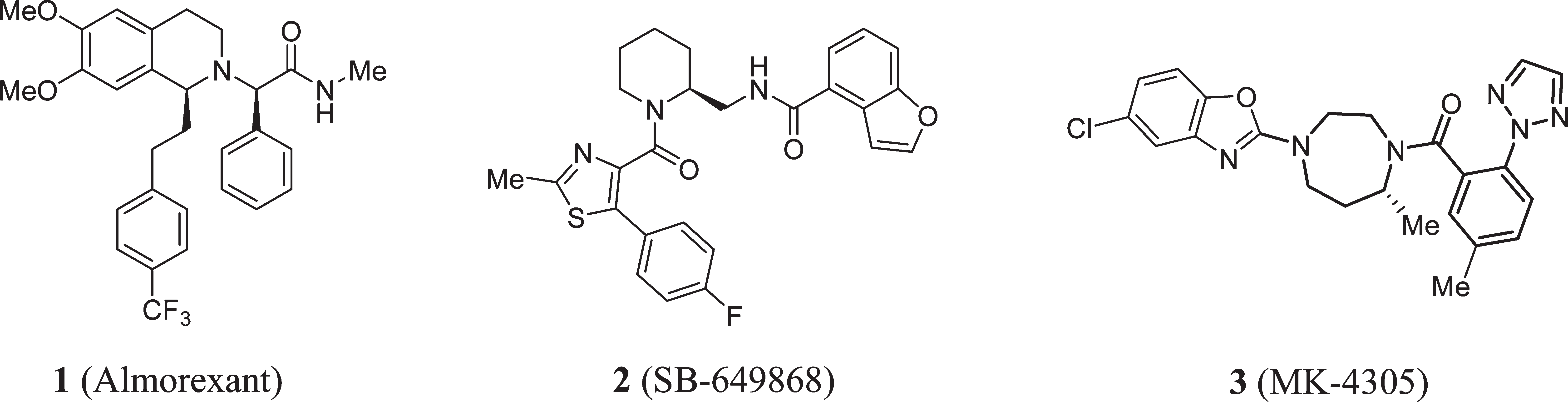
DORAs studied to date in clinical trials.