Chemicals list & Research Gallery
CAS number: 13450-90-3
Gallium trichloride appears as colorless needles. Used as a raw material in the production of metallic gallium and in the processing of mono crystal semiconductor compounds.
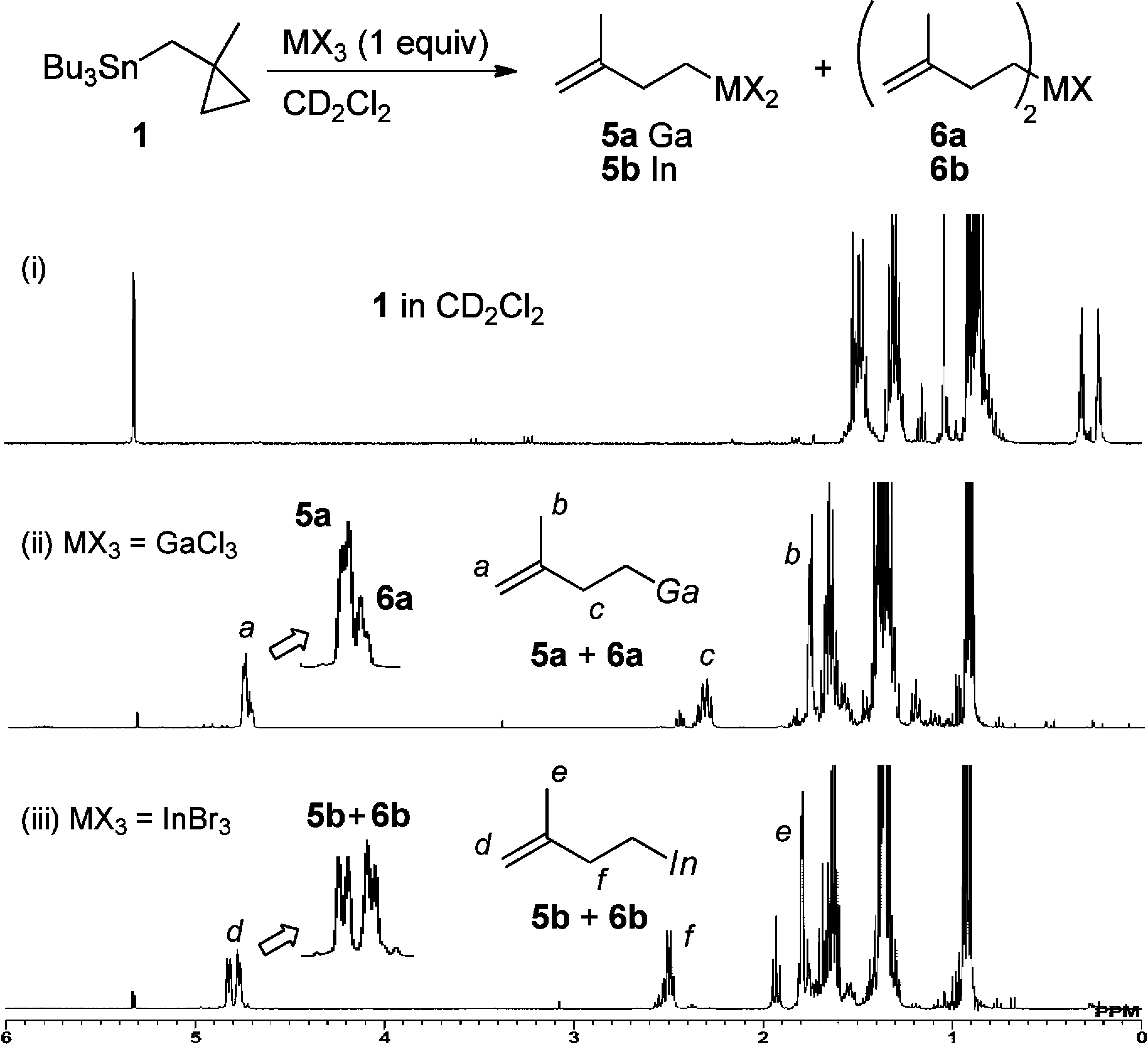
1H NMR spectra of (i) 1, (ii) the mixture of GaCl3 and 1, and (iii) the mixture of InBr3 and 1 in CD2Cl2.
CAS number: 13463-67-7
Titanium dioxide (TiO2) is considered as an inert and safe material and has used in many applications for decades.
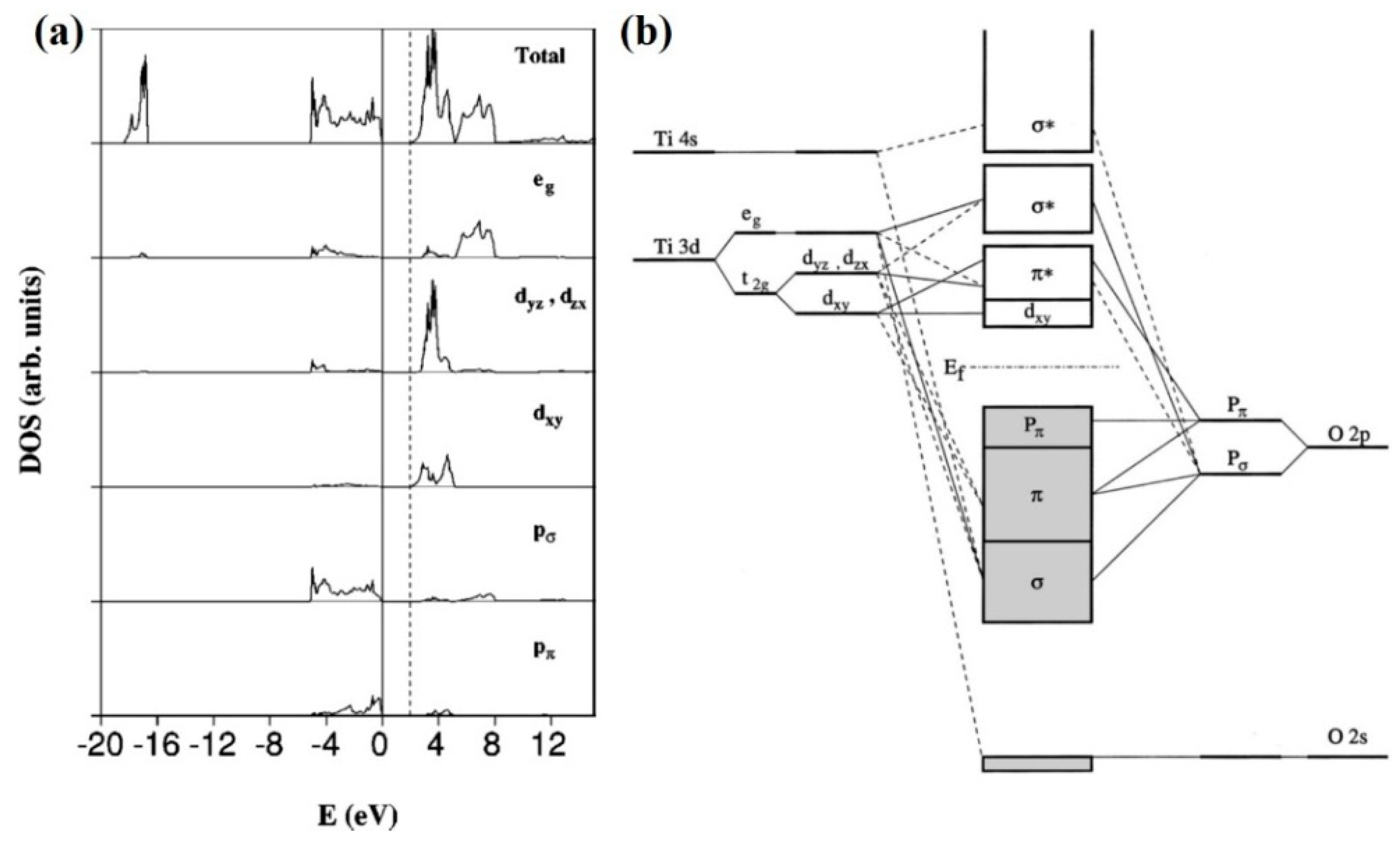
(a) Total and projected densities of states (DOS) of the anatase TiO2 structure and (b) molecular orbital bonding structure for anatase TiO2. Copyright 2004 The American Physical Society.
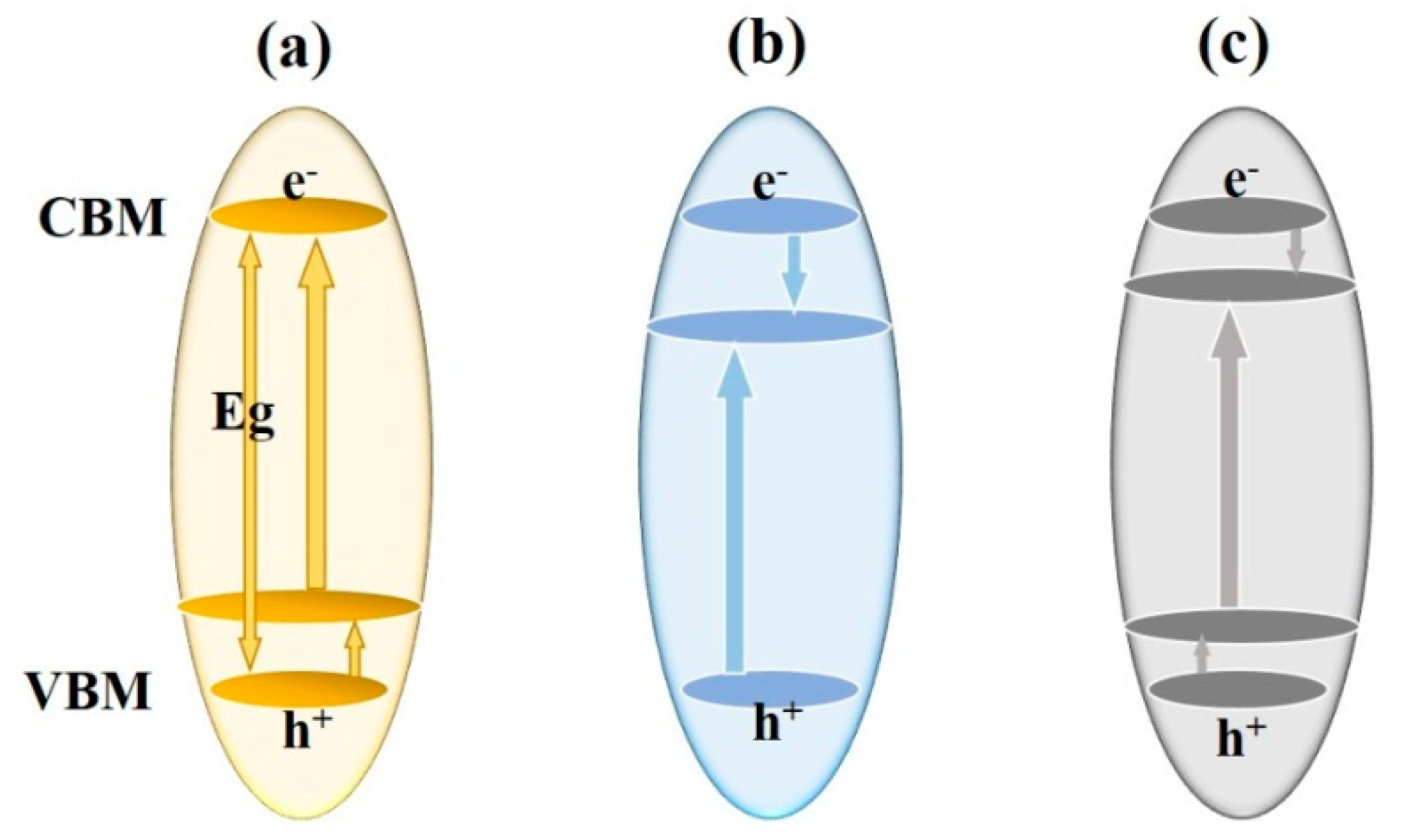
Three schemes of the band gap modifications of TiO2 match the solar spectrum: (a) a higher shift in valence band maximum (VBM); (b) a lower shift in conduction band minimum (CBM); and (c) continuous modification of both VBM and CBM.

TiO2 nanoparticles with different doping elements.
CAS number: 13465-09-3
Indium bromide (InBr3) is a chemical compound composed of indium and bromine. It's a white, crystalline powder that is also known as indium(III) bromide or indium tribromide. InBr3 is a Lewis acid, meaning it can accept an electron pair, and it finds use in organic synthesis and as a catalyst.

1H NMR spectra of (i) 1, (ii) the mixture of GaCl3 and 1, and (iii) the mixture of InBr3 and 1 in CD2Cl2.
CAS number: 134933-23-6
11-beta-methoxycurvularin is a macrolide.
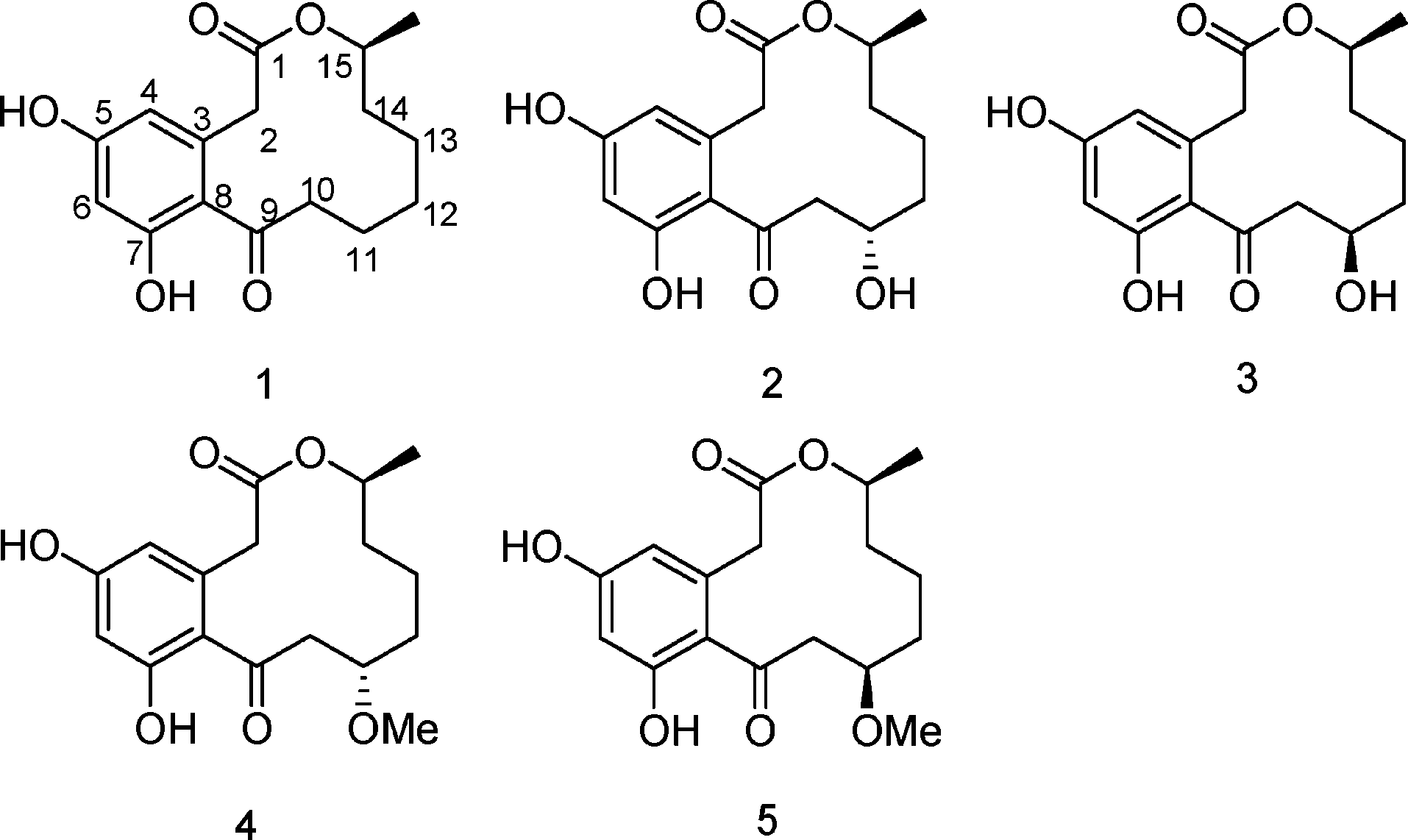
Curvularin (1), 11-α-hydroxycurvularin (2), 11-β-hydroxycurvularin (3), 11-α-methoxycurvularin (4), and 11-β-methoxycurvularin (5).
CAS number: 135-07-9
A thiazide diuretic with properties similar to those of hydrochlorothiazide.
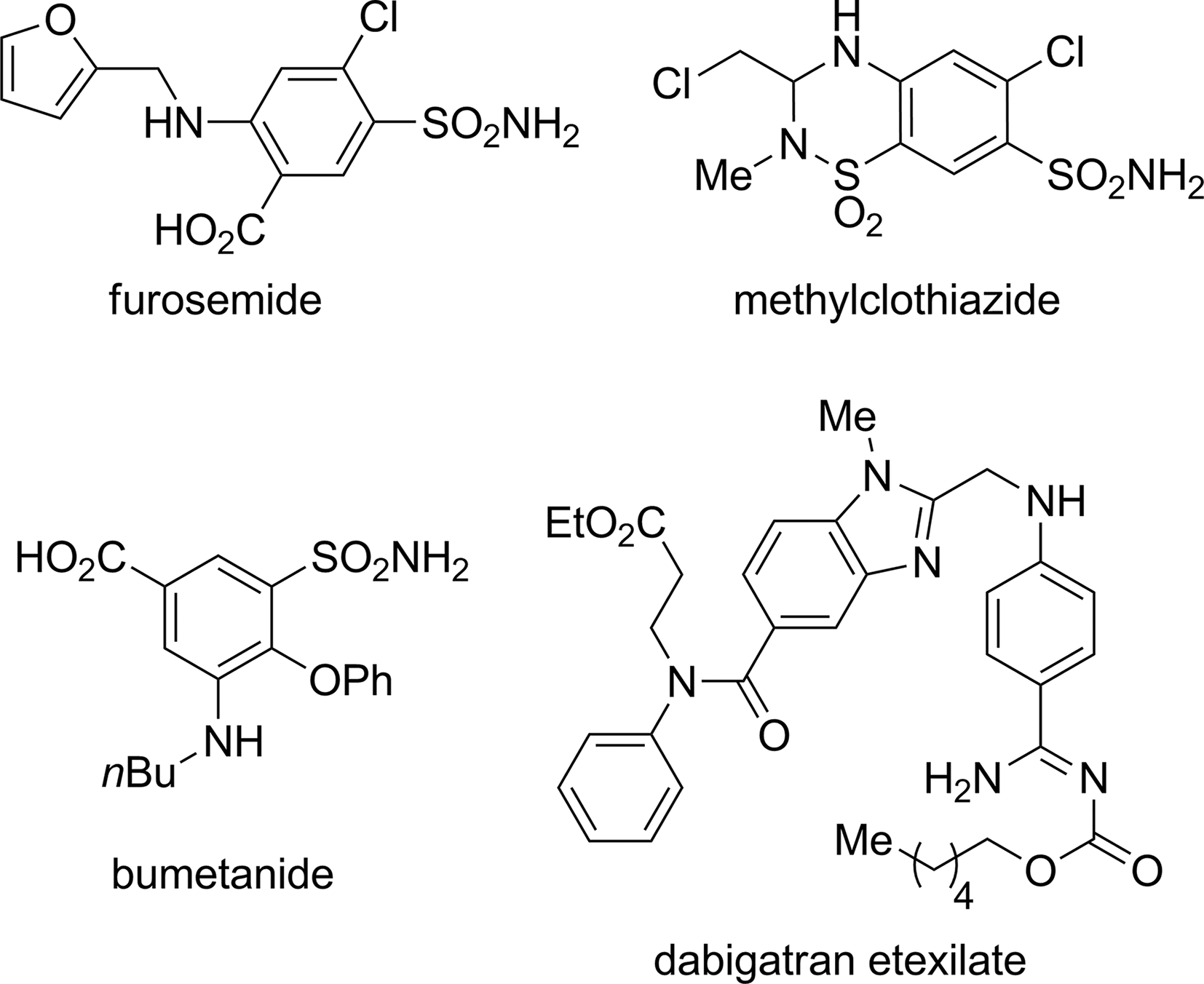
Some aniline-containing pharmaceuticals: furosemide, methylclothiazide, bumetanide
CAS number: 135-19-3
2-naphthol is a naphthol carrying a hydroxy group at position 2. It has a role as an antinematodal drug, a genotoxin, a human xenobiotic metabolite, a mouse metabolite, a human urinary metabolite and a radical scavenger.

Reaction scope of the three-component condensation of 2-naphthol, dimedone, and aldehyde in the presence of Fe3O4@SiO2-IL-ZnxCly.
CAS number: 135383-60-7
(S)-Dolaphenine HCl is a component of Dolastatin 10, an anti-tumor agent that inhibits tubulin polymerization.
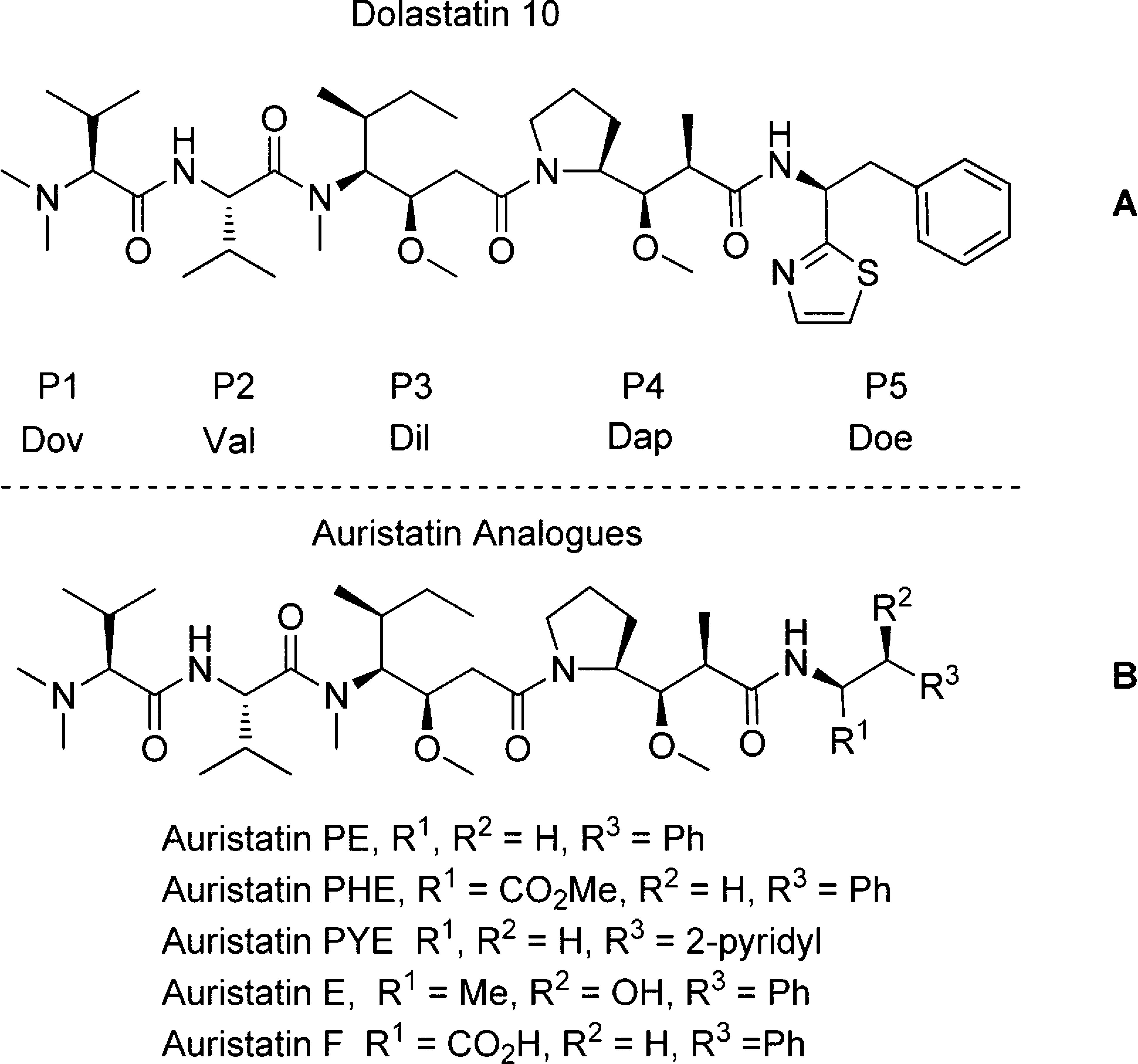
Dolastatin derivatives. Dov, dolavaline; Dil, (3R,4S,5S)- dolaisoleuine; Dap, (2R,3R,4S)-dolaisoleuine; Doe, (S)-dolaphenine.
CAS number: 135525-78-9
HIV-1 reverse transcriptase inhibitor
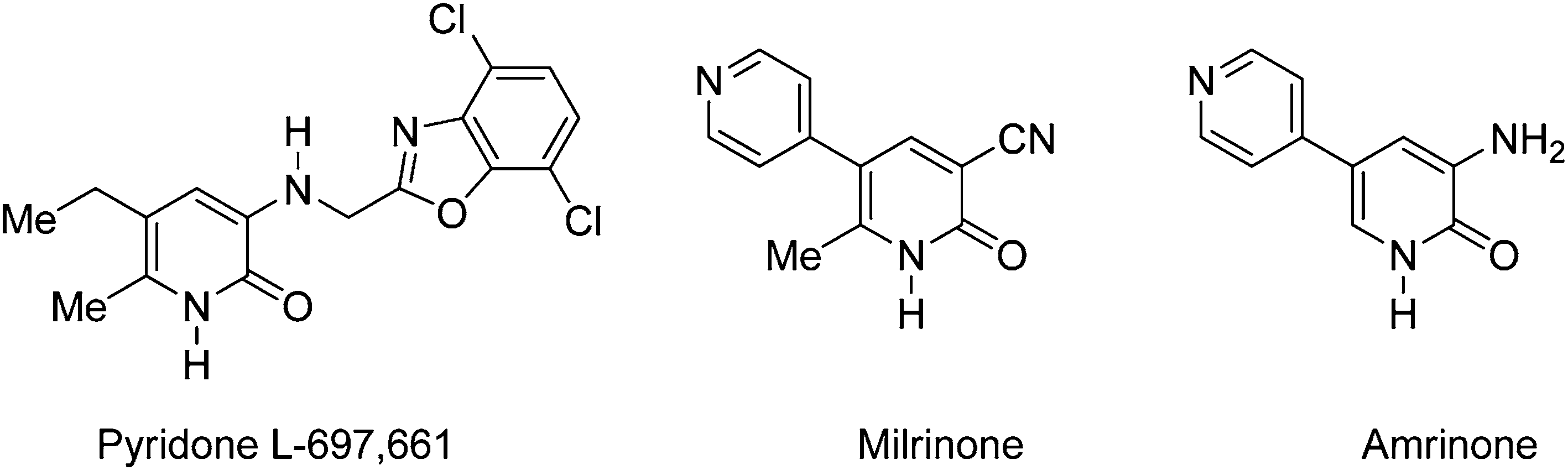
2-Pyridones possessing pharmacological properties: Amrinone, milrinone, L-697,661
CAS number: 13598-36-2
Phosphorous acid appears as a white or yellow crystalline solid (melting point 70.1 deg C) or a solution of the solid. Density 1.651 g /cm3. Contact may severely irritate skin, eyes, and mucous membranes. Toxic by ingestion, inhalation and skin absorption.
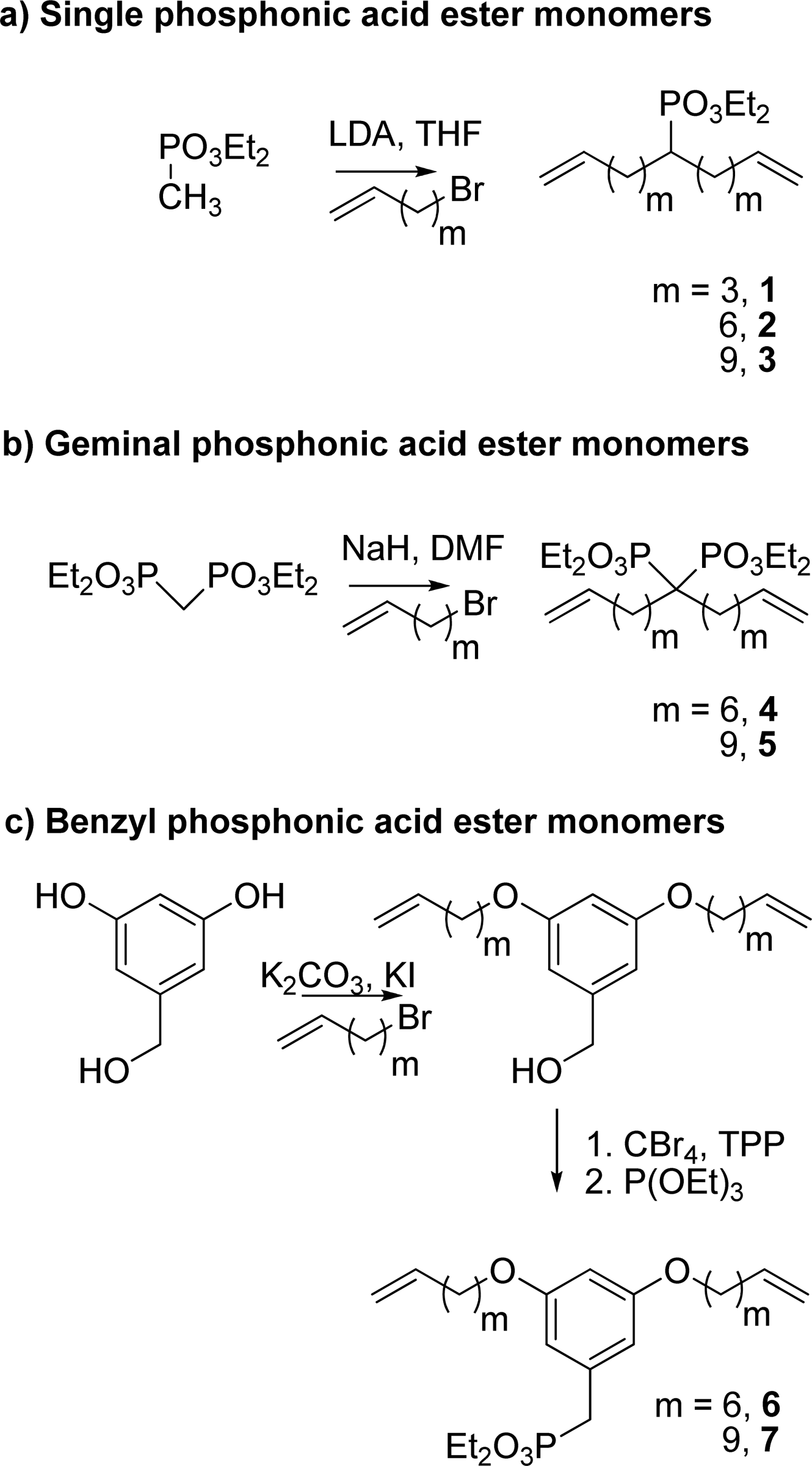
Efficient monomer Syntheses (phosphonic acid)
CAS number: 136-77-6
Hexylresorcinol is a substituted dihydroxybenzene. It exhibits antiseptic, anthelmintic, and local anesthetic properties. It can be found in topical applications for minor skin infections and in oral solutions or throat lozenges for pain relief and first aid antiseptic. The compound may also be used commonly in various commercial cosmetic anti-aging creams while ongoing studies research the possibility of using hexylresorcinol as an anti-cancer therapy - indications all of which require further study and testing at the current moment.
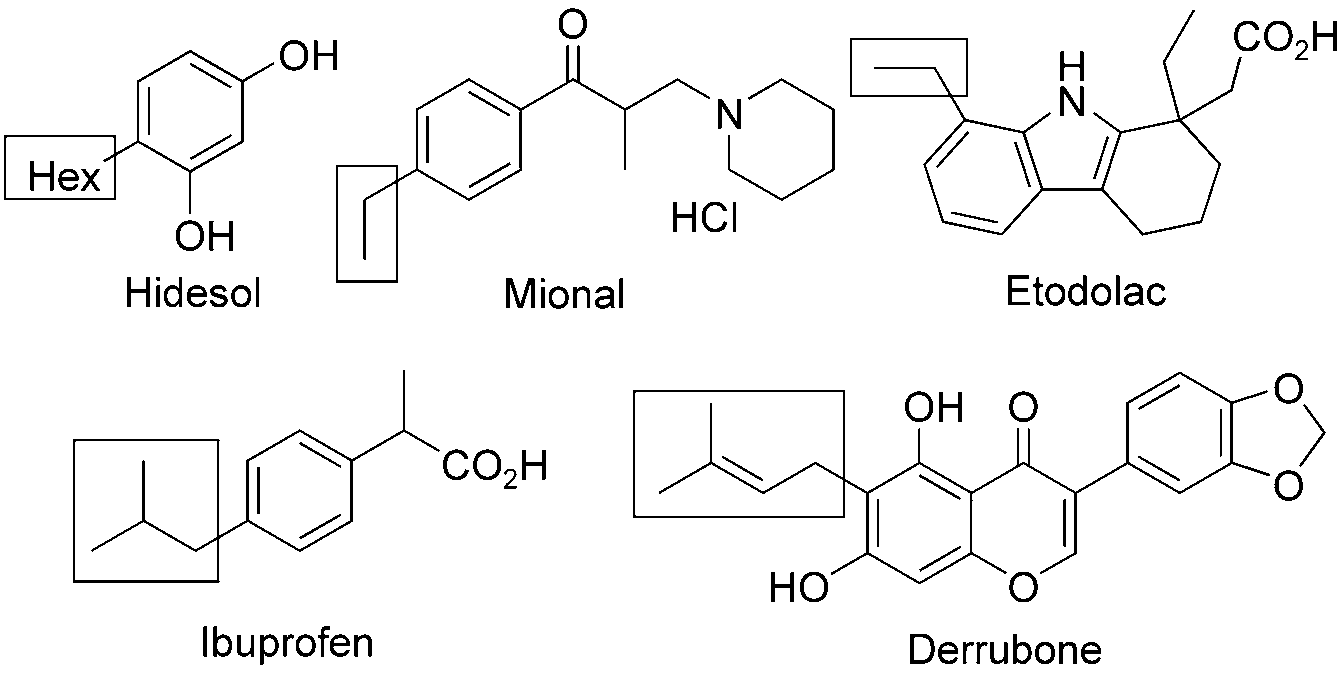
Drugs and bioactive natural products with alkyl side-chains: Hidesol, Mional, Etodolac, Derrubone