Chemicals list & Research Gallery
CAS number: 148608-52-0
Archaeosine is a 7-deazaguanine ribonucleoside having 7-formamidino-7-deazaguanine as the nucleobase. It is found in the majority of archaeal tRNAs specifically at position 15 of the dihydrouridine loop (D-loop), a position not modified in either eukaryotic or bacterial tRNA. It is functionally related to a 7-formamidino-7-deazaguanine.
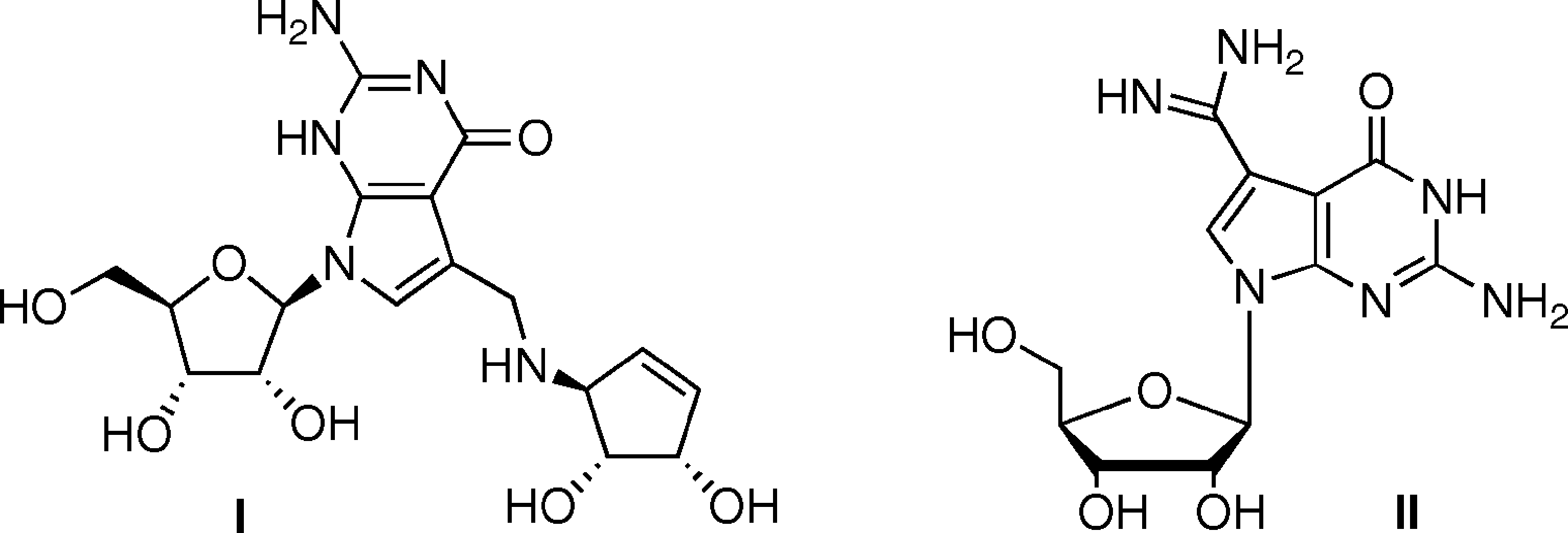
Queuosine (I) and Archaeosine (II).
CAS number: 148700-85-0
(2S,3S)-3-[[3,5-bis(trifluoromethyl)phenyl]methoxy]-2-phenylpiperidine is a member of piperidines.
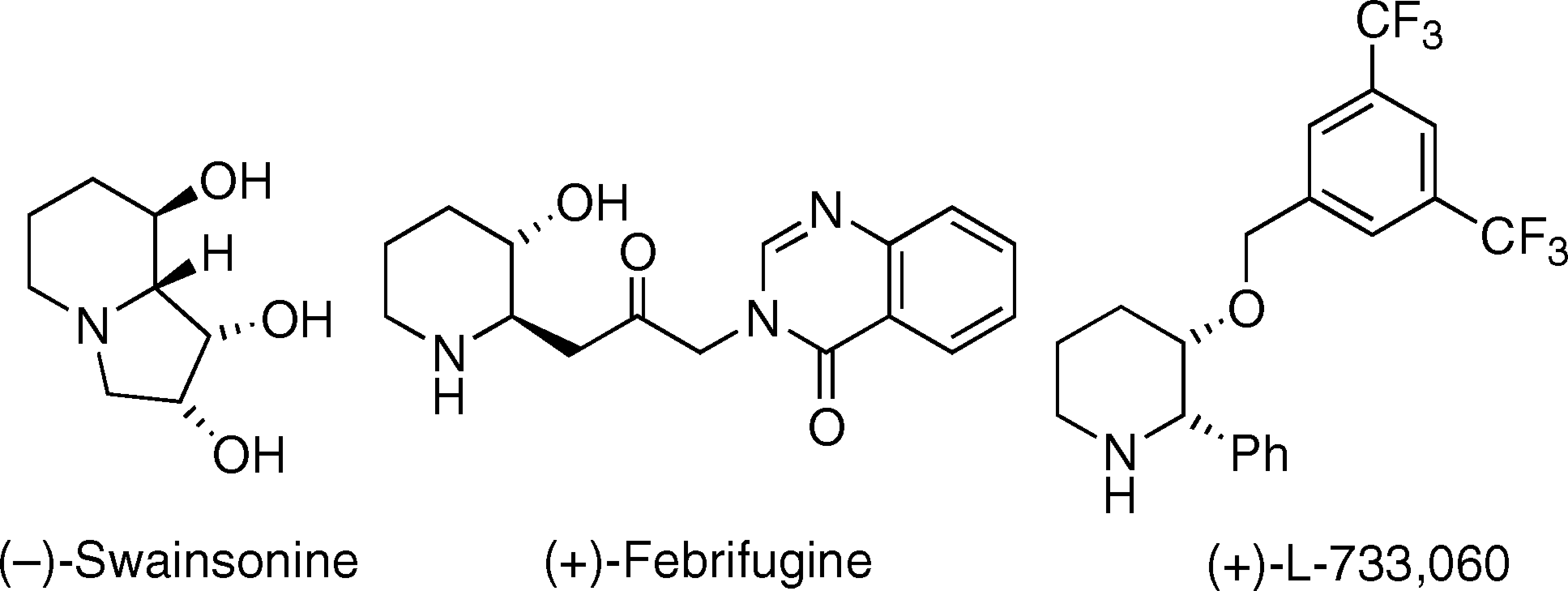
Biologically active β-hydroxy piperidines.
CAS number: 14875-96-8
Heme is a molecule containing iron that is crucial for many biological processes, particularly oxygen transport.
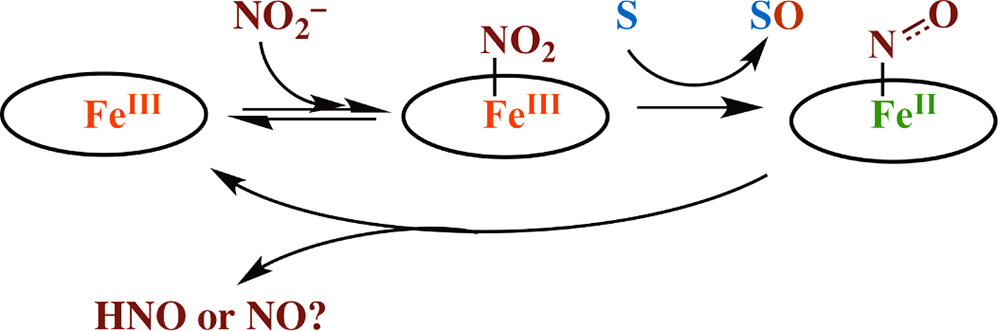
Nitrite Reduction Mediated by Heme Models. Routes to NO and HNO?
CAS number: 148893-10-1
HATU is a highly efficient and widely used coupling reagent in peptide synthesis and organic chemistry. It facilitates the formation of amide bonds by activating carboxylic acids for nucleophilic attack by amines, often leading to high yields and minimal racemization. HATU is a uronium-type reagent that incorporates 7-azabenzotriazole as the leaving group, which enhances its reactivity compared to other coupling agents like HBTU or DCC. Its structure includes a uronium core with tetramethyl substitution and a hexafluorophosphate (PF₆⁻) counterion, contributing to its stability and solubility in polar organic solvents such as DMF and DMSO. Due to its high efficiency, HATU is particularly favored in solid-phase peptide synthesis (SPPS) and other applications requiring reliable amide bond formation.
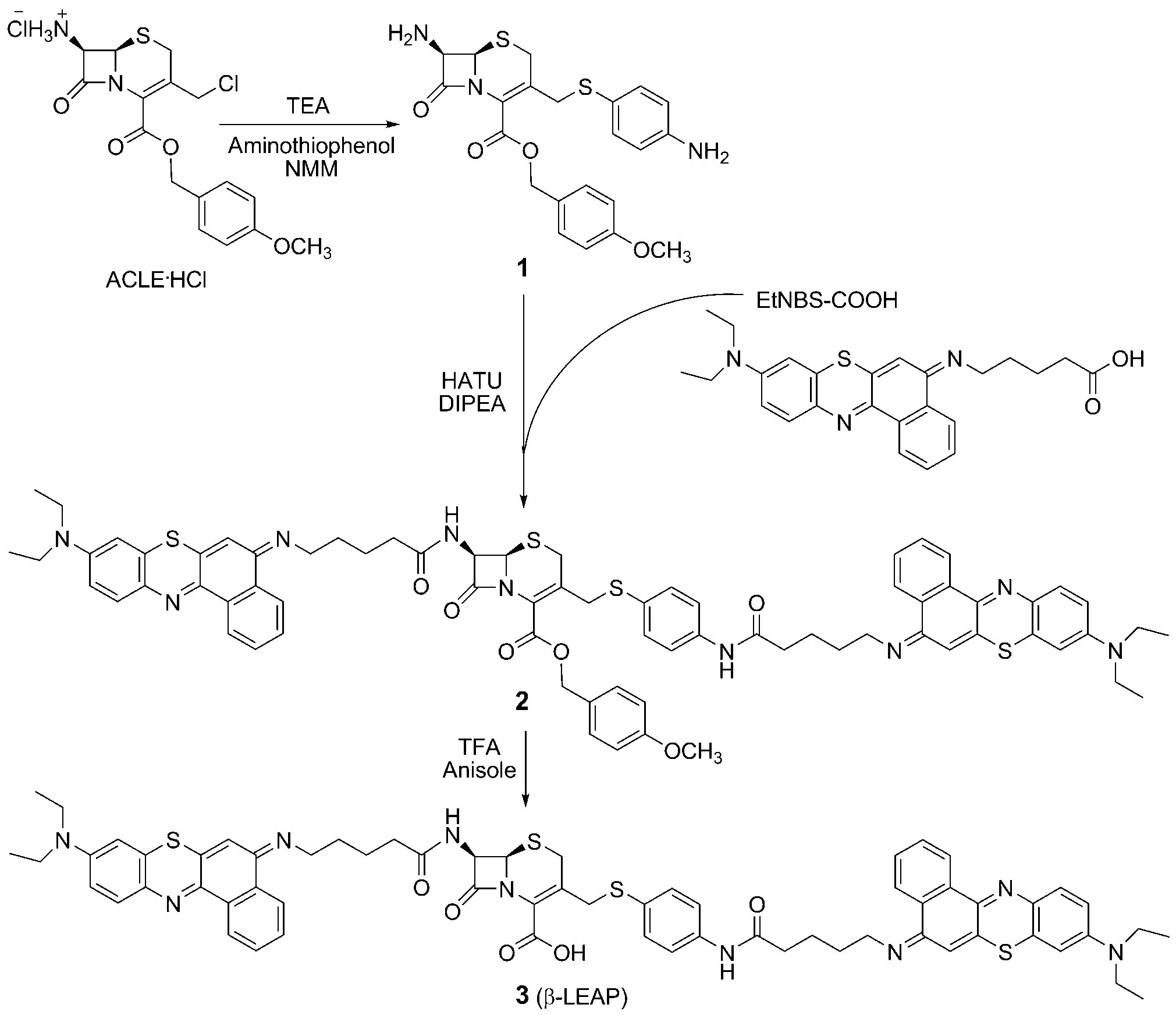
Synthesis of β-LEAP. DIPEA=N,N’-diisopropylethylamine, NMM=4-methylmorpholine, HATU=O-(7-azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate, TEA=triethylamine, TFA=trifluoroacetic acid.
CAS number: 149-30-4
2-Mercaptobenzothiazole is 1,3-Benzothiazole substituted at the 2-position with a sulfanyl group. It has a role as a carcinogenic agent and a metabolite. It is a member of benzothiazoles and an aryl thiol.
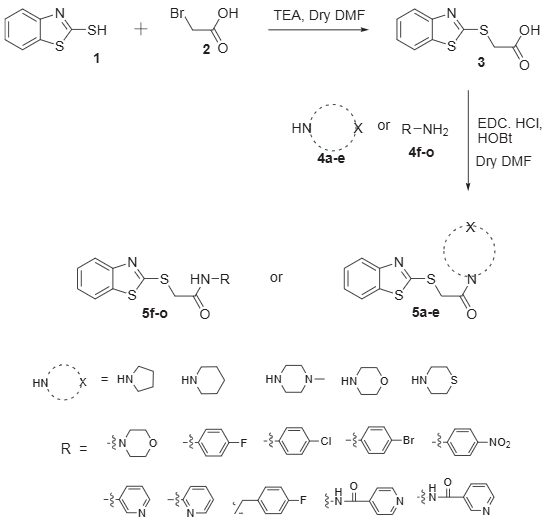
Synthetic approach for 2-mercaptobenzothiazole conjugates.
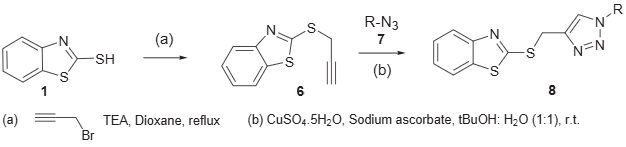
Synthesis of 1,2,3-triazole conjugates of 2-mercaptobenzothiazole.
CAS number: 149-91-7
Gallic acid is a trihydroxybenzoic acid in which the hydroxy groups are at positions 3, 4, and 5. It has a role as an astringent, a cyclooxygenase 2 inhibitor, a plant metabolite, an antioxidant, an antineoplastic agent, a human xenobiotic metabolite, an EC 1.13.11.33 (arachidonate 15-lipoxygenase) inhibitor, an apoptosis inducer and a geroprotector. It is a conjugate acid of a gallate.
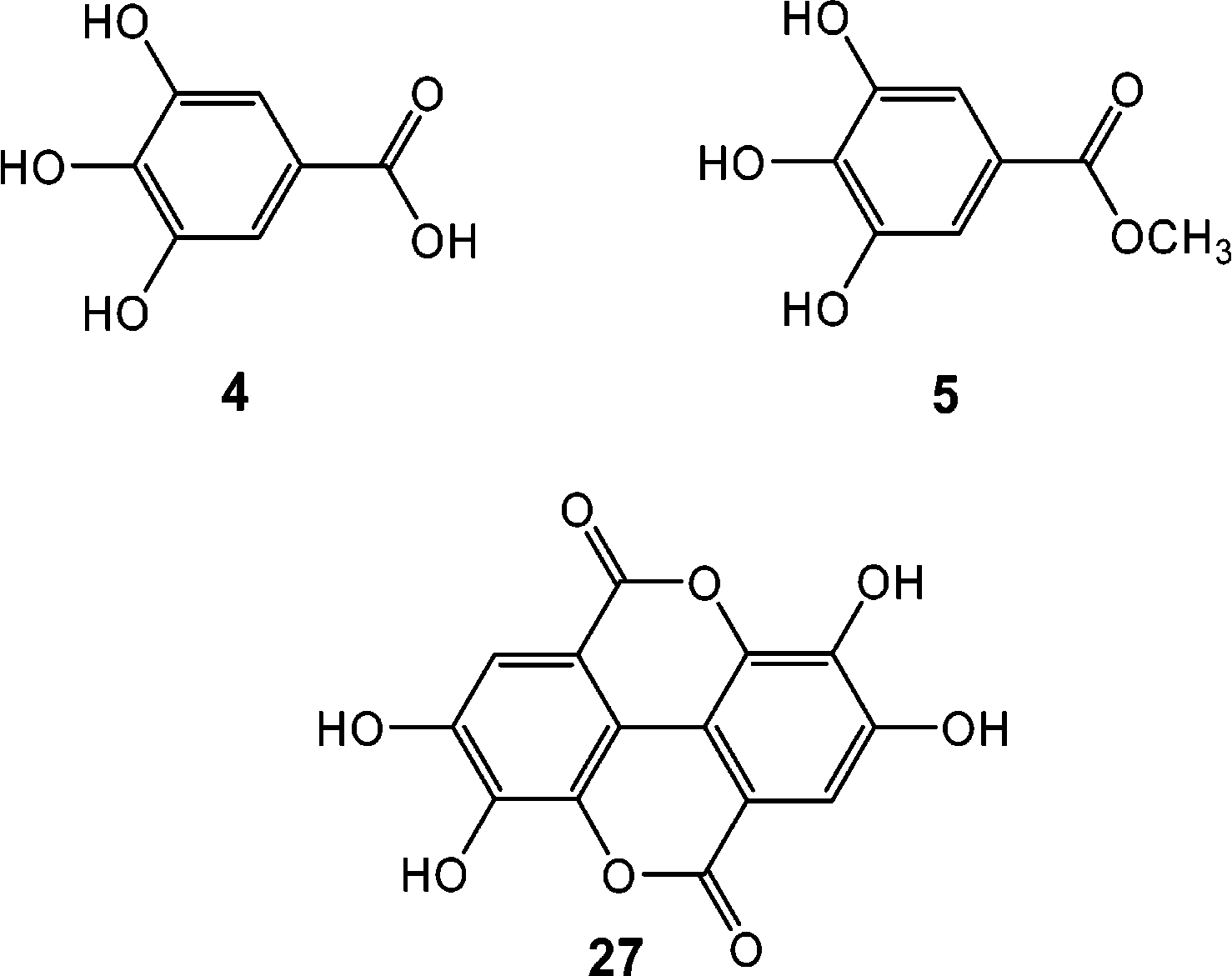
Structure of Commercially Available Gallic Acid Derivatives
CAS number: 149488-17-5
Trovirdine is a thiourea non-nucleoside reverse transcriptase inhibitor.
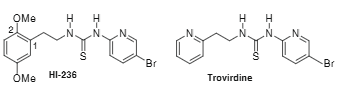
Structures of HI-236 and Trovirdine.
CAS number: 149647-78-9
Vorinostat is a drug that has been approved by the U.S. Food and Drug Administration (FDA) under the brand name Zolinza for the treatment of a certain type of cancer.Vorinostat is also being studied as an investigational drug as part of a strategy to cure HIV infection.As an investigational HIV drug, vorinostat belongs to a group of drugs called latency-reversing agents.
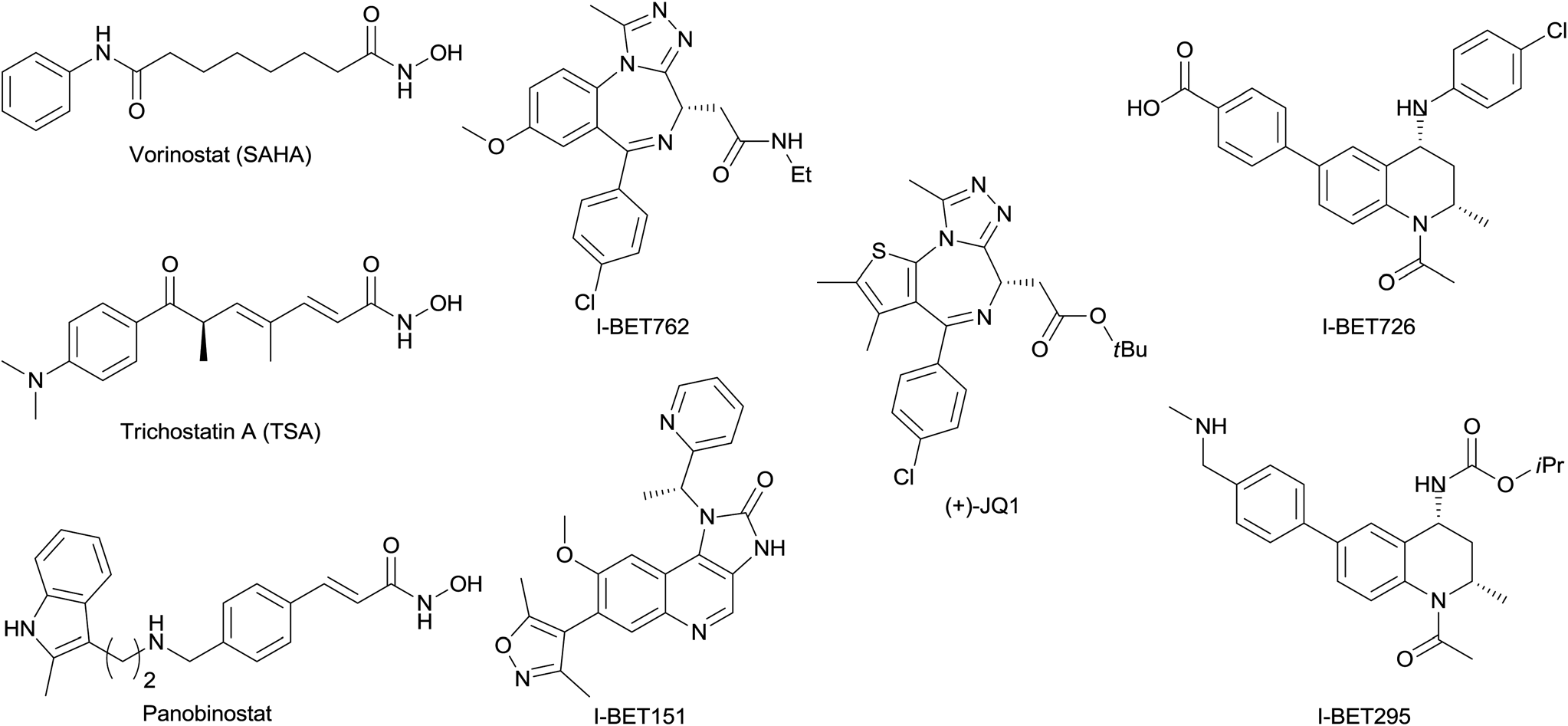
Structures of selected published BET inhibitors and selected hydroxamic acid HDAC inhibitors.
CAS number: 1499-10-1
9,10-diphenylanthracene is a member of the class of anthracenes that is anthracene in which both of the hydrogens on the central ring are substituted by phenyl groups. It has a role as a fluorochrome and a photosensitizing agent.
![Plots of the fluorescence quantum yields determined in THF/H2O solutions by using 9,10-diphenylanthracene (F=90% in cyclohexane) as the internal standard versus the fraction of water in the solvent. Insets: photo- graphs of the TPE–Ar luminogens in the THF/water mixture (fw =99%) taken under the illumination of a 365 nm UV lamp. Excitation wavelengths [nm]: 320 for TPE–ptol and TPE–mtol, and 310 for the other six fluorophores.](http://www.wlxkc.cn/picture/3386517_12.png)
Plots of the fluorescence quantum yields determined in THF/H2O solutions by using 9,10-diphenylanthracene (F=90% in cyclohexane) as the internal standard versus the fraction of water in the solvent. Insets: photo- graphs of the TPE–Ar luminogens in the THF/water mixture (fw =99%) taken under the illumination of a 365 nm UV lamp. Excitation wavelengths [nm]: 320 for TPE–ptol and TPE–mtol, and 310 for the other six fluorophores.
CAS number: 151-51-9
Carbodiimide is a commonly used water loss agent, mainly used to activate carboxyl groups to promote the formation of amides and esters.
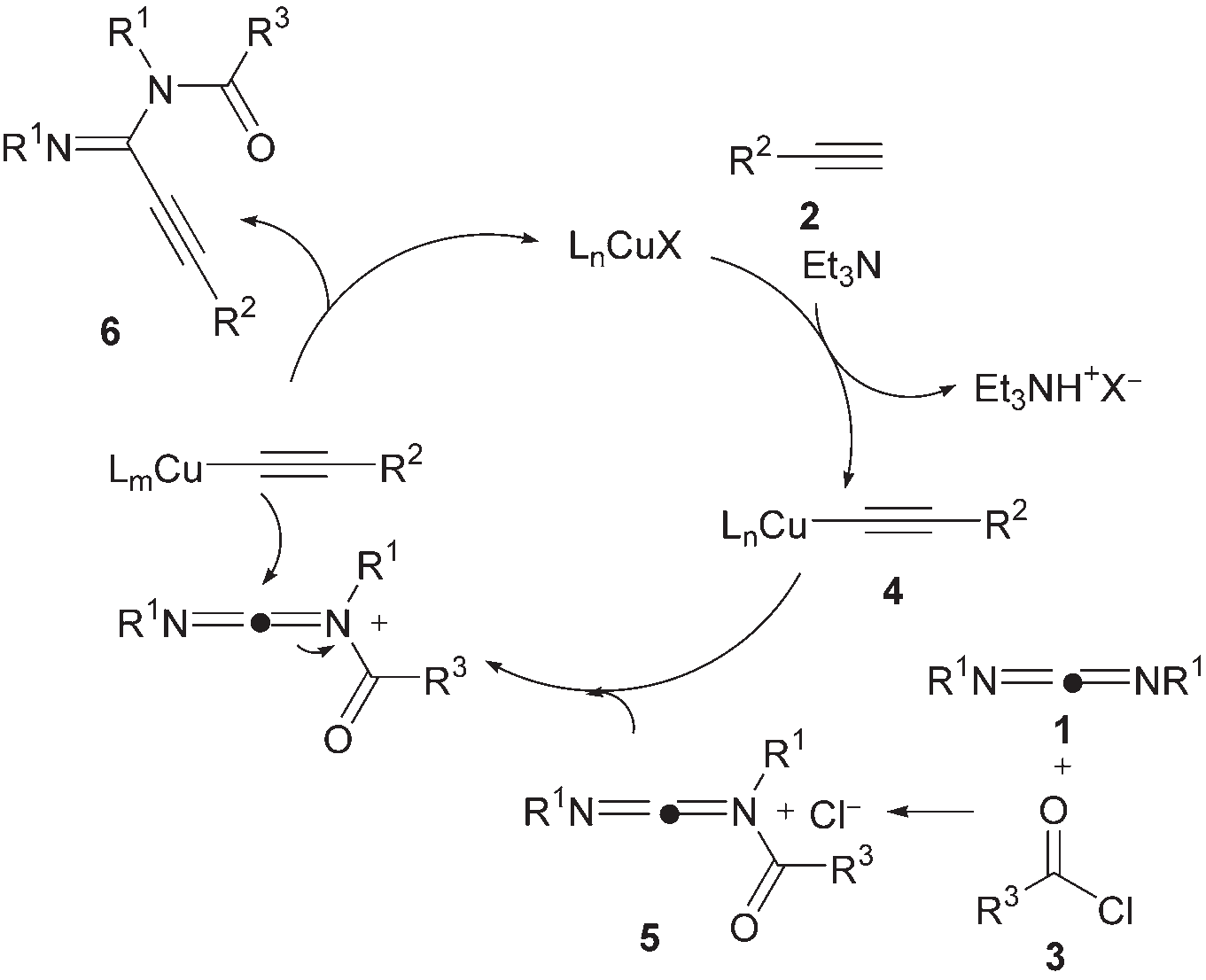
Carbodiimide 1 reacts with acyl chloride 3 to generate N-acylimide salt 5. Alkyne 2 is immediately converted to acetylide copper 4 in the presence of triethylamine, and 1 equivalent of triethylamine acid salt is released. Subsequently, 4 undergoes nucleophilic attack on 5 to obtain the target product 6, and the copper catalyst is released, completing the catalytic cycle.
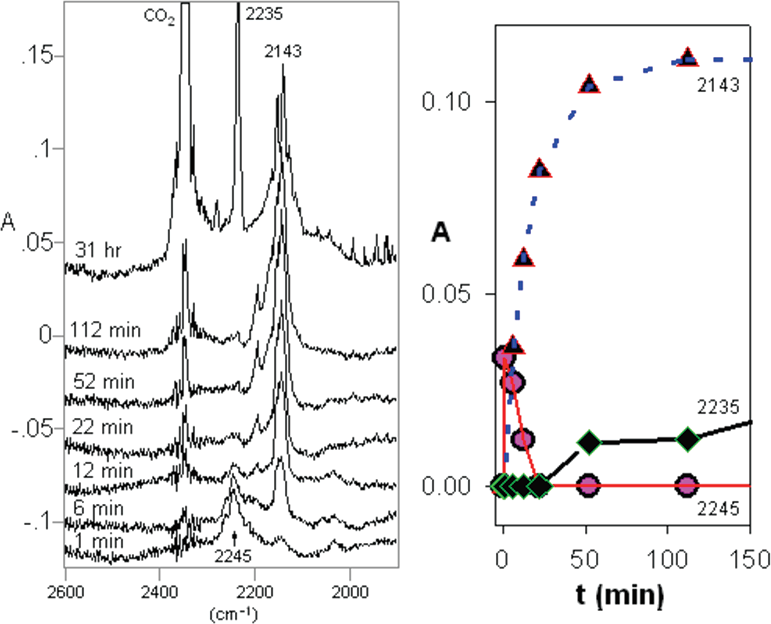
Partial IR difference spectra of 5-phenyl-2-(trimethylsilyl)-tetrazole 22 at 12 K in Ar matrix at different photolysis times at 254 nm, showing peaks due to photolysis products. Abscissa 1900−2600 cm−1. (Right) Plots of IR absorbance of different wavenumbers versus photolysis time from 0 to 150 min. The 2245 and 2143 cm−1 bands are assigned to nitrile imine 23 and carbodiimide 25, respectively, and the 2235 cm−1 band is assigned to benzonitrile.