Chemicals list & Research Gallery
CAS number: 1002127-43-6
Raphanuside, chemically known as (2S,4aS,6R,7S,8S,8aR)-2-(4-hydroxy-3,5-dimethoxyphenyl)-6-(hydroxymethyl)-3,4a,6,7,8,8a-hexahydro-2H-pyrano[3,2-b][1,4]oxathiine-7,8-diol, is a naturally occurring glycoside isolated from radish (Raphanus sativus) seeds. It features a complex polycyclic structure combining a pyran ring fused with an oxathiine ring, substituted with a hydroxymethyl group and two hydroxyl groups at positions 7 and 8, as well as a 4-hydroxy-3,5-dimethoxyphenyl moiety at position 2.
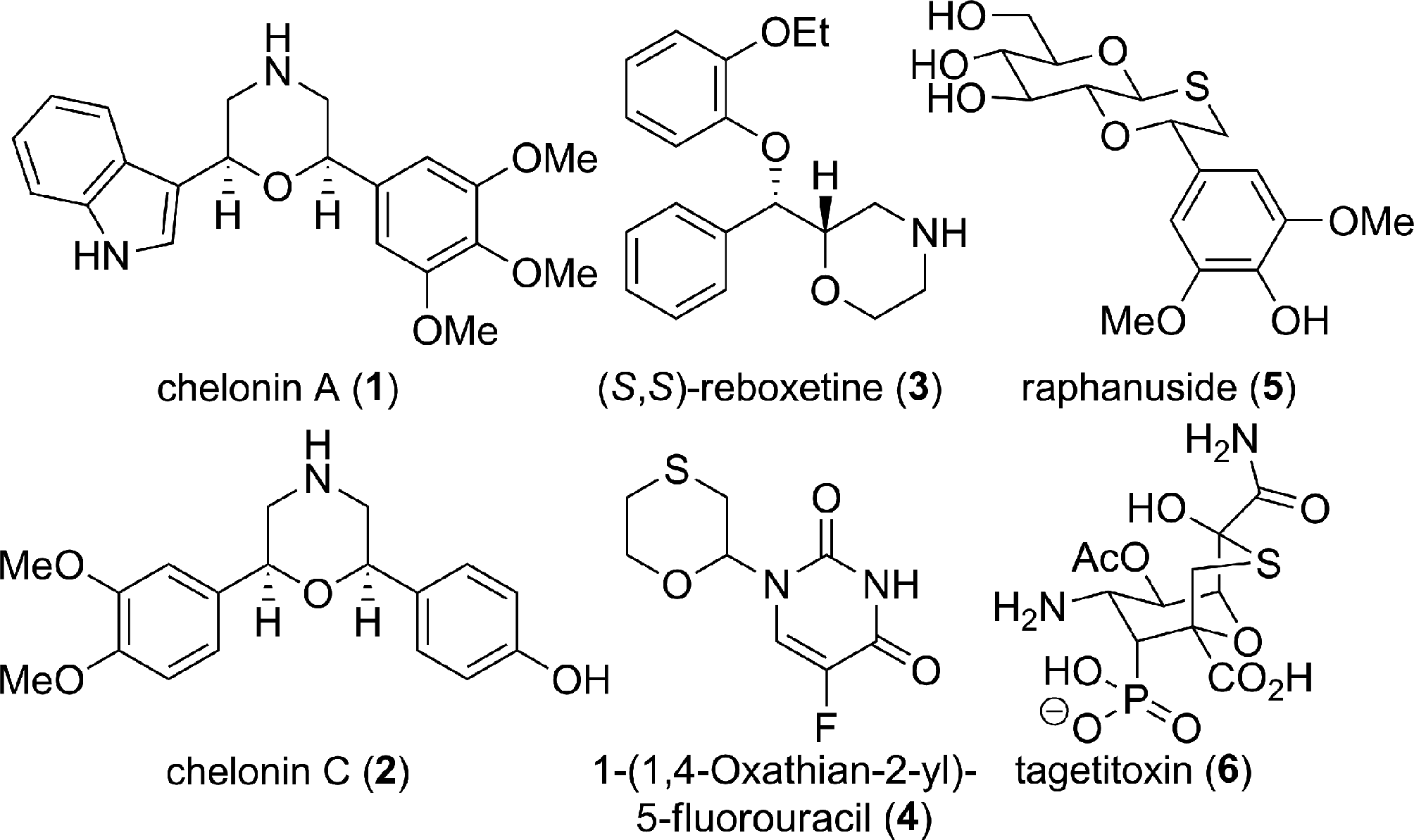
Morpholines and 1,4-oxathiane ring bearing natural products and bioactive molecules.
CAS number: 1003-29-8
Pyrrole-2-carboxaldehyde is a pyrrole carrying a formyl substituent at the 2-position. It is a member of pyrroles and a 1,3-thiazole-2-carbaldehyde.
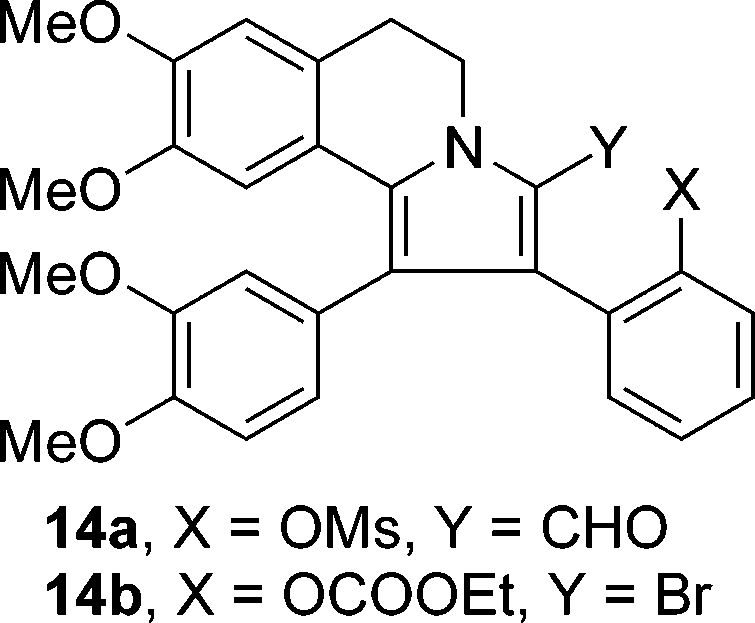
Structure of 2-formylpyrrole 14a and 2-bromopyrrole 14b.
CAS number: 1005045-39-5
LSM-33280 is an amidobenzoic acid.
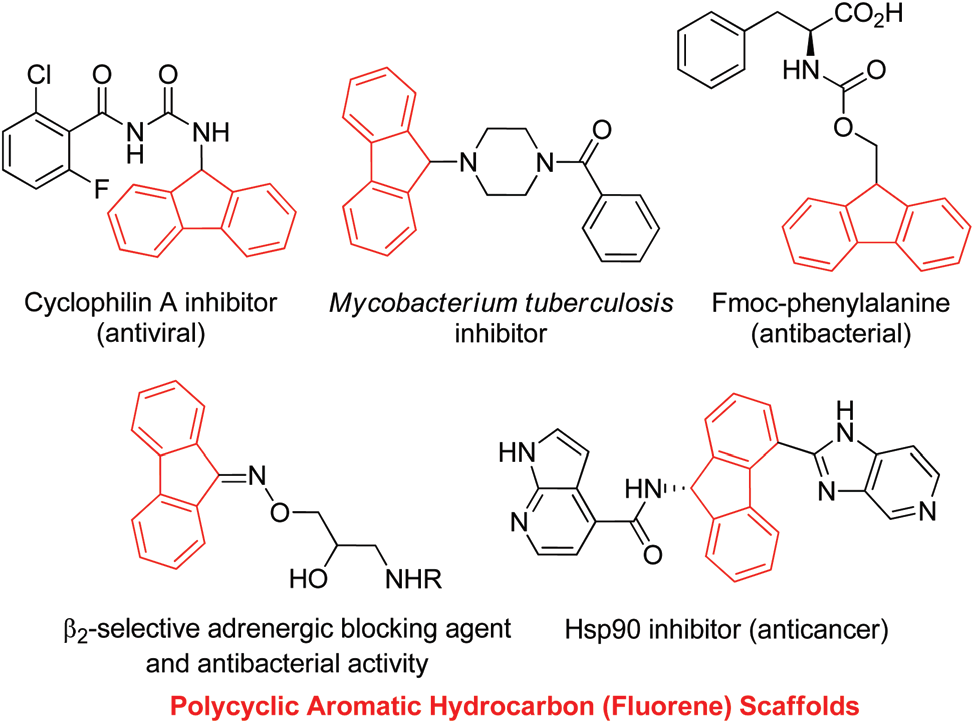
Selected examples of pharmaceuticals and bioactive compounds that contain polycyclic aromatic hydrocarbon (PAH) scaffolds: Cyclophilin A inhibitor, Mycobacterium Tuberculosis Inhibitor, FMOC-PHENYLALANINE, Hsp90 inhibitor. The fluorene core is shown in red.
CAS number: 1008-89-5
2-Phenylpyridine is a colorless, viscous liquid and a member of the phenylpyridine family. It's known for its use in the synthesis of fluorescent metal complexes, particularly those used in organic light-emitting diodes (OLEDs).
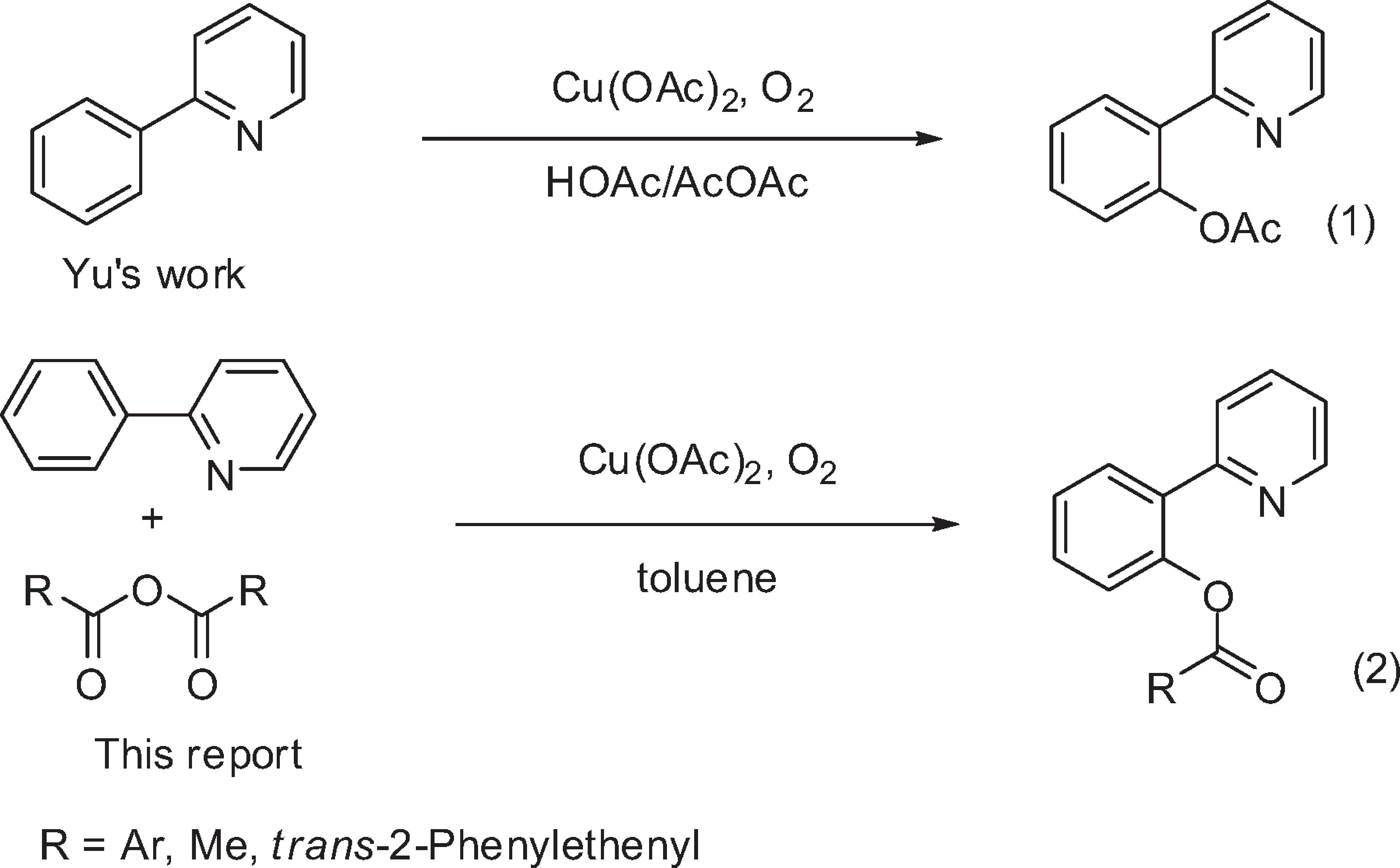
Copper-Catalyzed Acyloxylation of the 2-Phenylpyridine C-H Bond
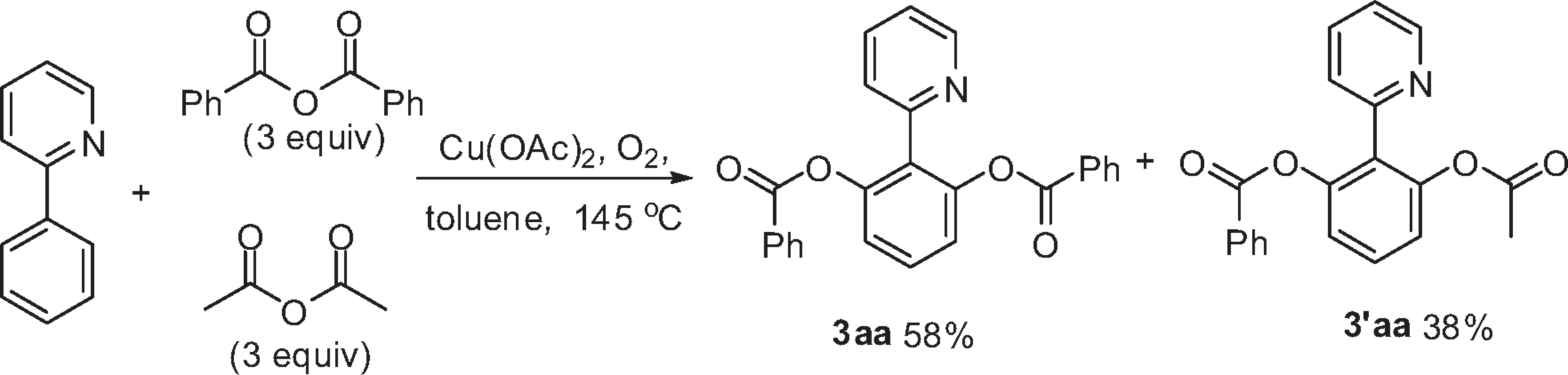
Competition Reaction of Anhydride with 2-Phenylpyridine
CAS number: 101-60-0
Porphyrin is a group of organic heterocyclic compounds that are comprised of four modified pyrrole subunits connected via methine bridges and form an aromatic macrocyclic structure, which has one or more side chains attached. Many porphyrins are naturally occurring pigments. Porphyrins with metal ions bound to the porphin ring can be cofactors for biological processes, such as oxygen transport by hemes and photosynthesis by chlorophyll.
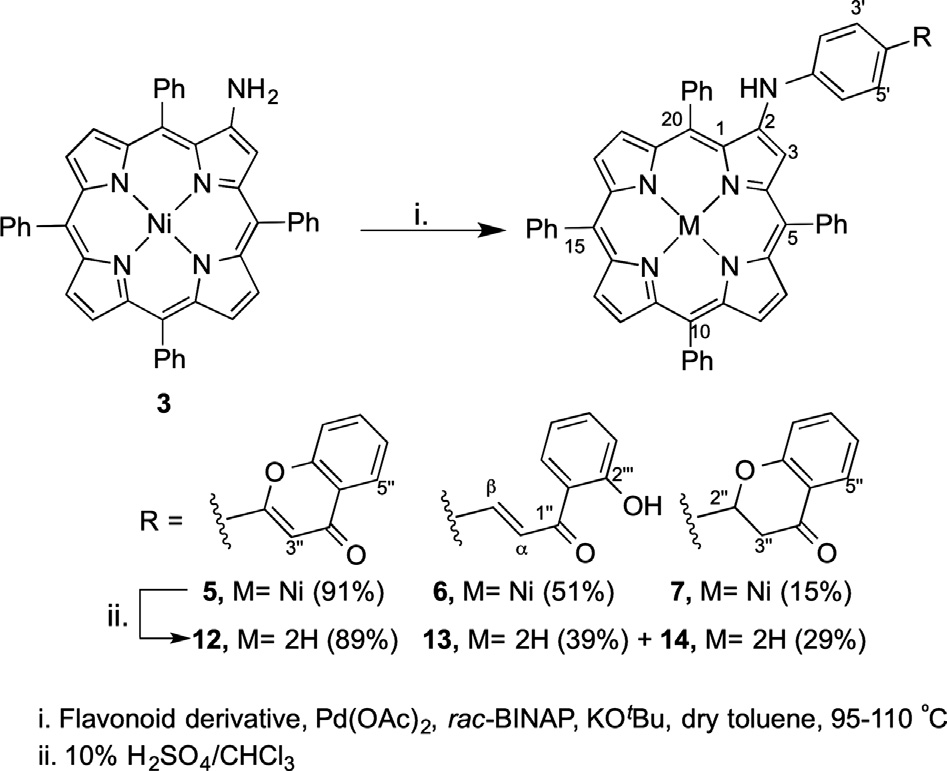
Synthetic strategy to prepare flavonoid–porphyrin conjugates at β-position.
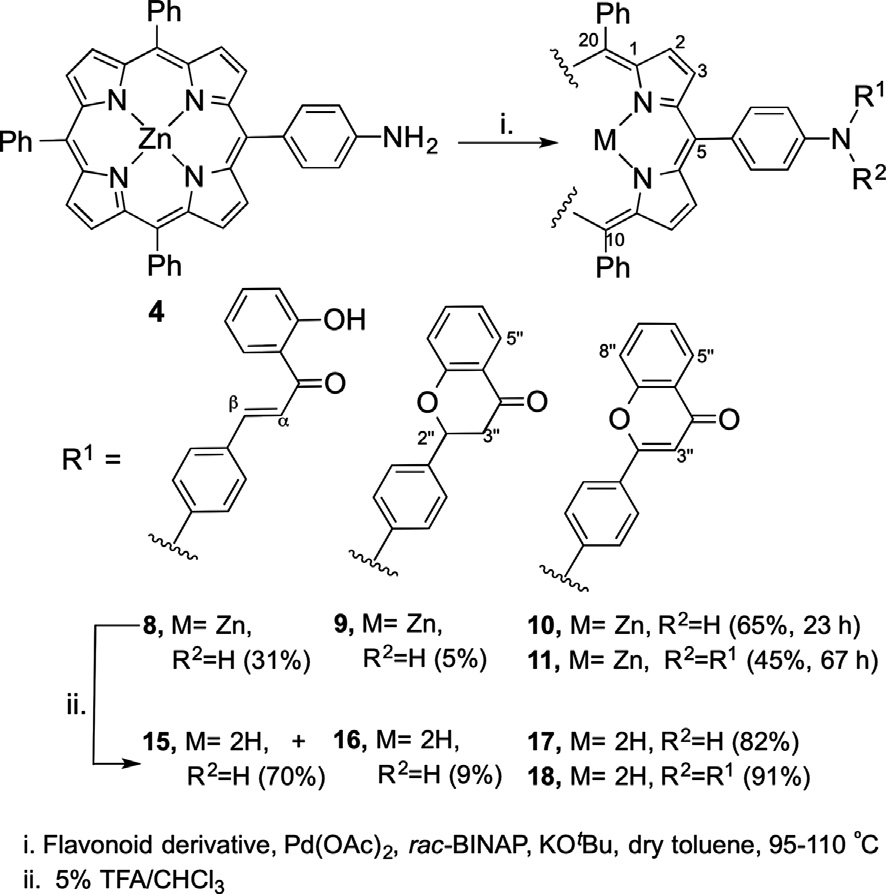
Synthetic strategy to prepare flavonoid–porphyrin conjugates at meso-position.
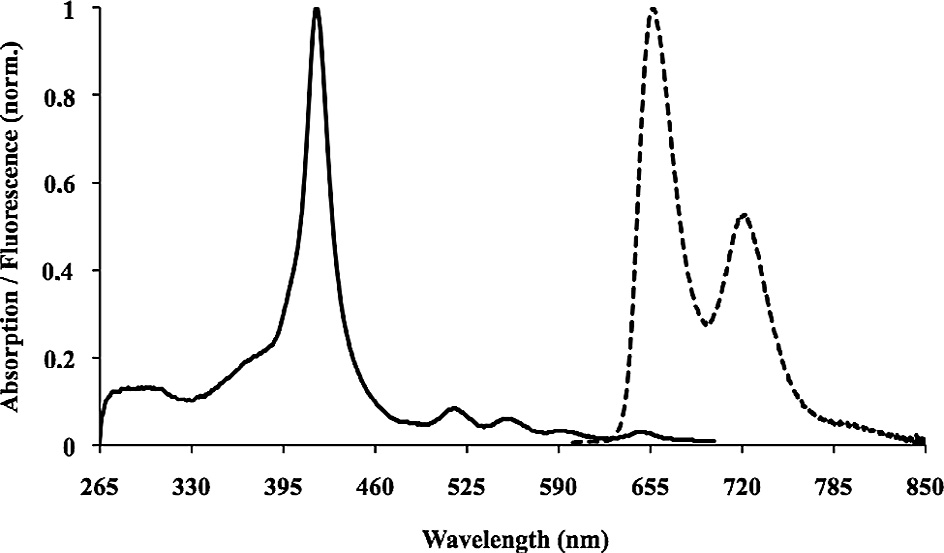
Representative normalized absorption (solid) and fluorescence spectra (dotted) (kexc = 550 nm, OD = 0.02) of flavonoid–porphyrin conjugate 17 in DMF.
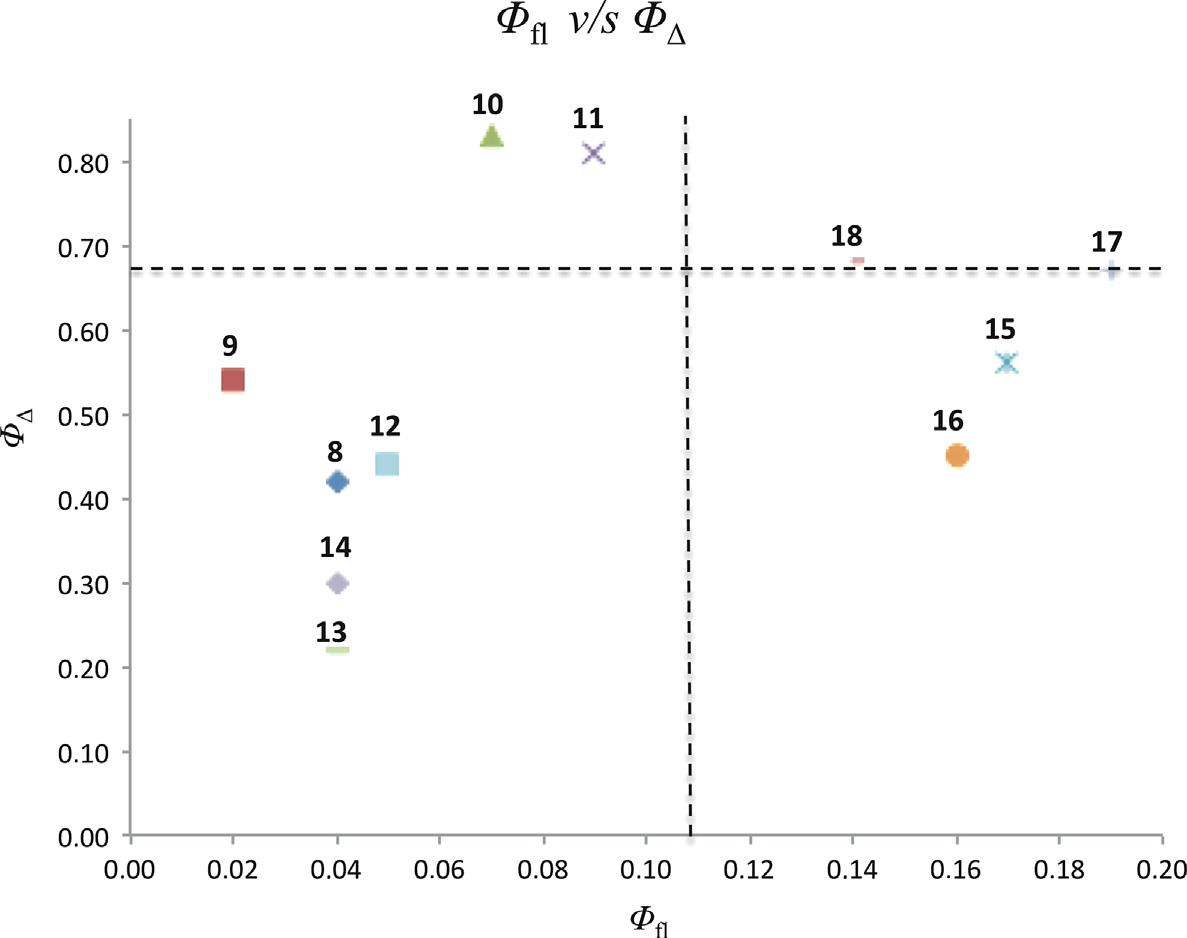
Plot of the singlet oxygen quantum yield (Ø△) of the flavonoid–porphyrin conjugates versus their fluorescence quantum yield (Øfl) in DMF. The interception of the dotted lines represents the value of the standard (TPP).
CAS number: 101-84-8
Diphenyl ether is an aromatic ether in which the oxygen is attached to two phenyl substituents. It has been found in muscat grapes and vanilla. It has a role as a plant metabolite.
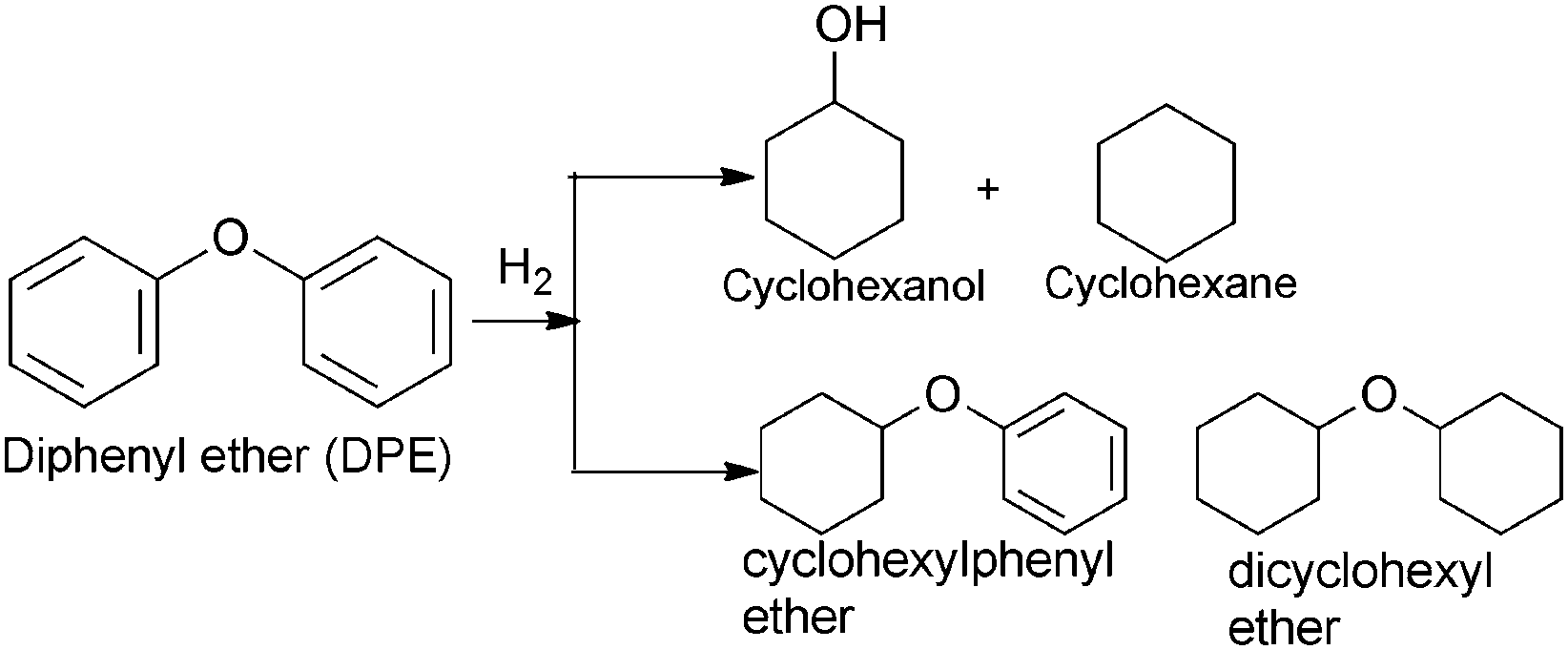
Possible reaction path of DPE hydrogenolysis–hydrogenation.
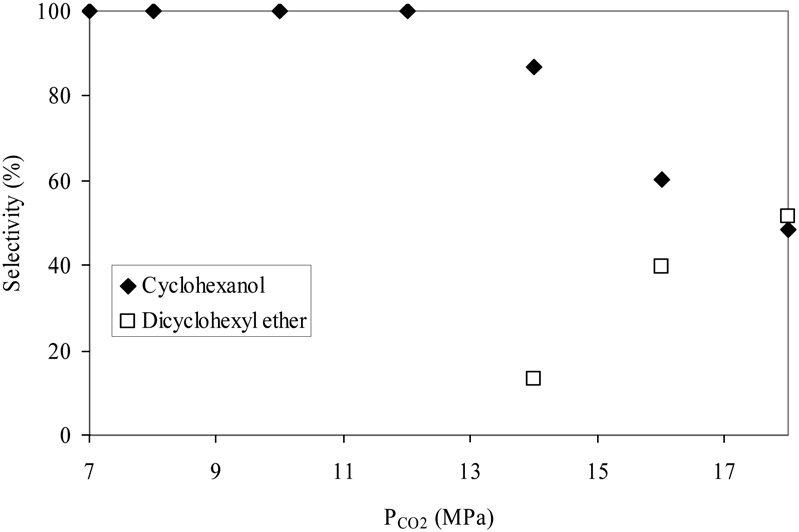
Effect of CO2 pressure on the hydrogenolysis–hydrogenation of DPE.
CAS number: 10139-51-2
Ceric Ammonium Nitrate is used as an efficient catalyst for ring opening of epoxides in the presence of water, thiols and acetic acid.
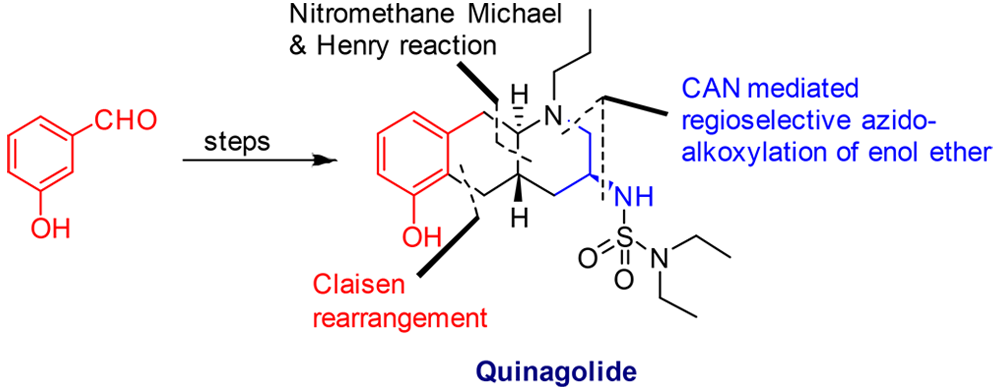
The total synthesis of ()-quinagolide was accomplished via a ceric ammonium nitrate (CAN)-mediated regioselective azidoalkoxylation route of enol ethers.
CAS number: 10140-70-2
(-)-Curvularin is an organic compound, specifically a macrocyclic lactone, known for its phytotoxic and anti-inflammatory properties. It's a secondary metabolite produced by various fungi, including those in the genera Curvularia, Penicillium, and Alternaria.
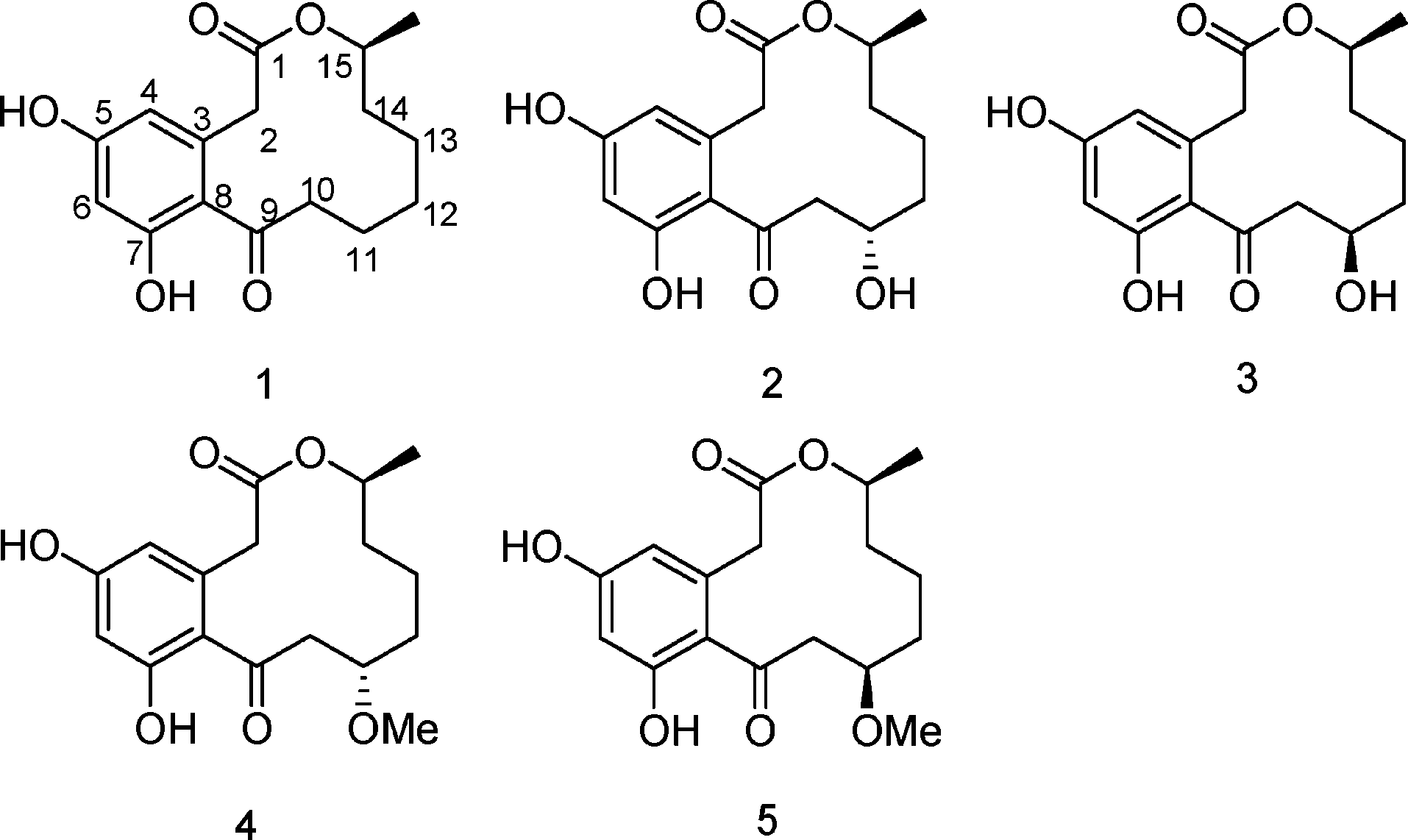
Curvularin (1), 11-α-hydroxycurvularin (2), 11-β-hydroxycurvularin (3), 11-α-methoxycurvularin (4), and 11-β-methoxycurvularin (5).
CAS number: 10167-11-0
3,4-Dihydro-1H-pyrimidin-2-one is a heterocyclic organic compound with a pyrimidine ring structure. It's a derivative of pyrimidine where the double bond between the 3rd and 4th carbon atoms has been reduced, and it has a keto group at the 2nd carbon position.
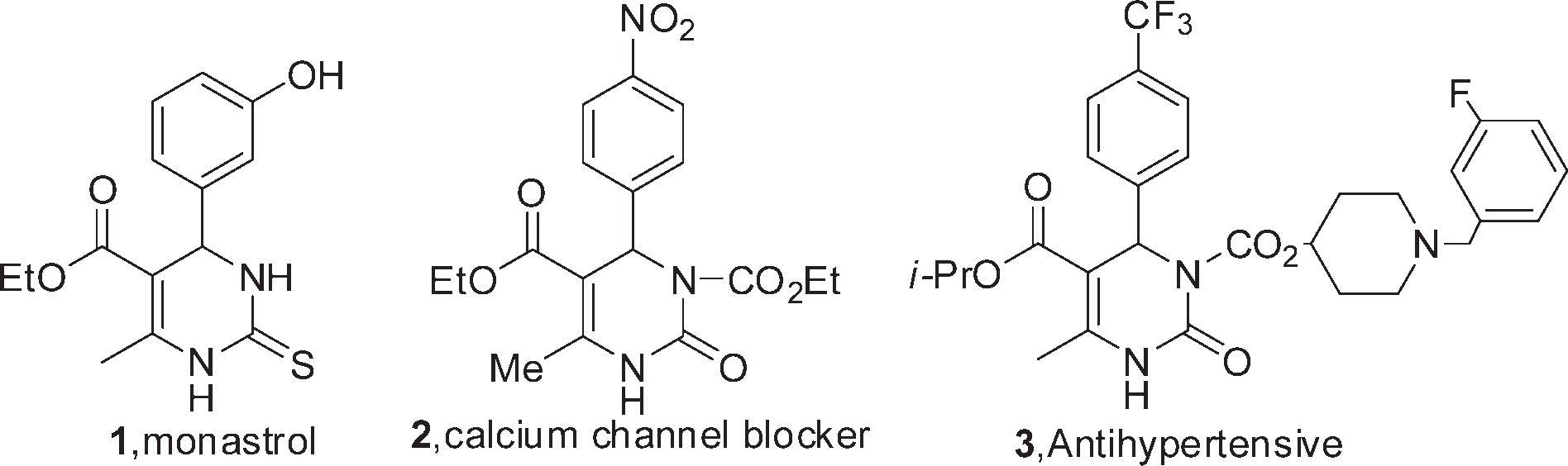
3,4-dihydropyrimidin-2(1H)-ones/thiones.
CAS number: 101932-71-2
Calyculin A from Discodermia calyx is a dual action toxin that blocks calcium influx and inhibits protein Ser/Thr phosphatases.
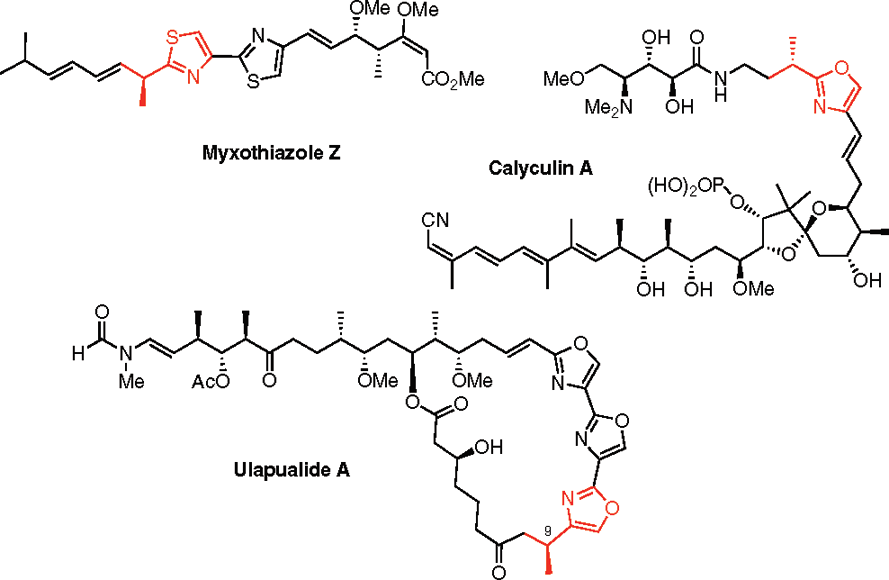
Structure of myxothiazole Z, calyculin A, and ulapualide A.