Chemicals list & Research Gallery
CAS number: 103177-37-3
Pranlukast is a cysteinyl leukotriene receptor-1 antagonist. It antagonizes or reduces bronchospasm caused, principally in asthmatics, by an allergic reaction to accidentally or inadvertently encountered allergens.
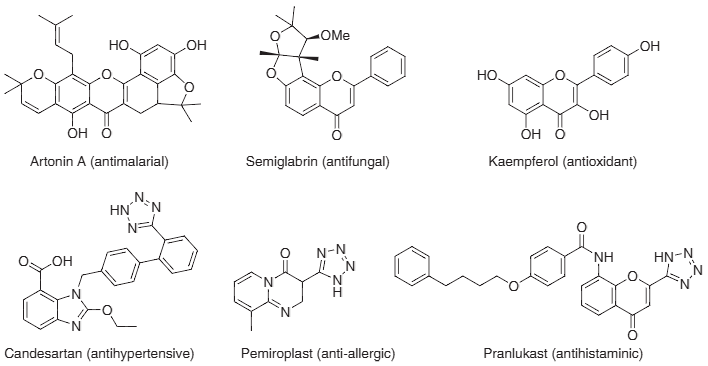
Naturally occurring flavones and pharmacologically important tetrazole drugs: Artonin A, Semiglabrin, Kaempferol, Candesartan, Pemiroplast, Pranlukast.
CAS number: 103577-45-3
Prevacid, also known as lansoprazole, is a Proton Pump Inhibitor. The mechanism of action of lansoprazole is as a Proton Pump Inhibitor. The physiologic effect of lansoprazole is by means of Inhibition Gastric Acid Secretion.
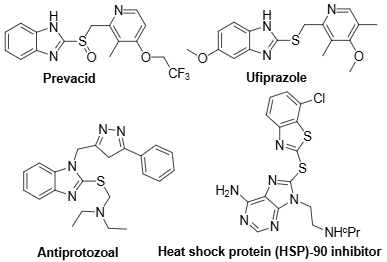
Some biologically active benzimidazole sulfides: Prevacid, Ufiprazole, Antiprotozoal, Heat shock protein (HSP)-90 inhibitor.
CAS number: 103619-04-1
Invictolide is a chemical compound that plays a significant role in the social behavior of red imported fire ants (Solenopsis invicta). It is one of three queen recognition pheromones produced by the queens of this species.
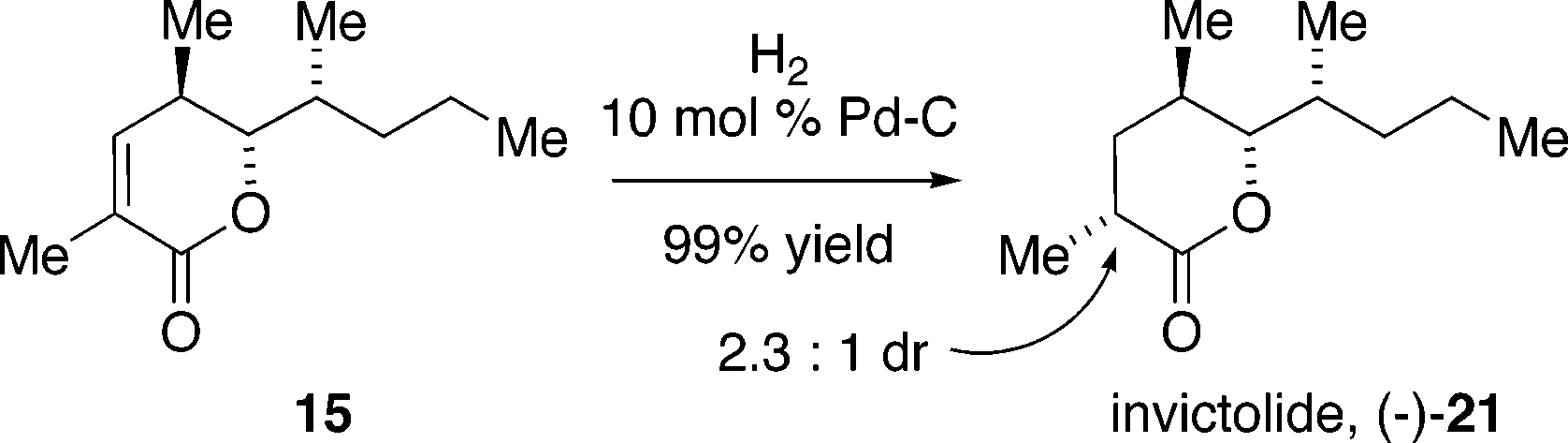
A Short Synthesis of Invictolide
CAS number: 103782-08-7
Allosamidin, a chitinase inhibitor produced by. Streptomyces, acts as an inducer of chitinase production in. its producing strain.
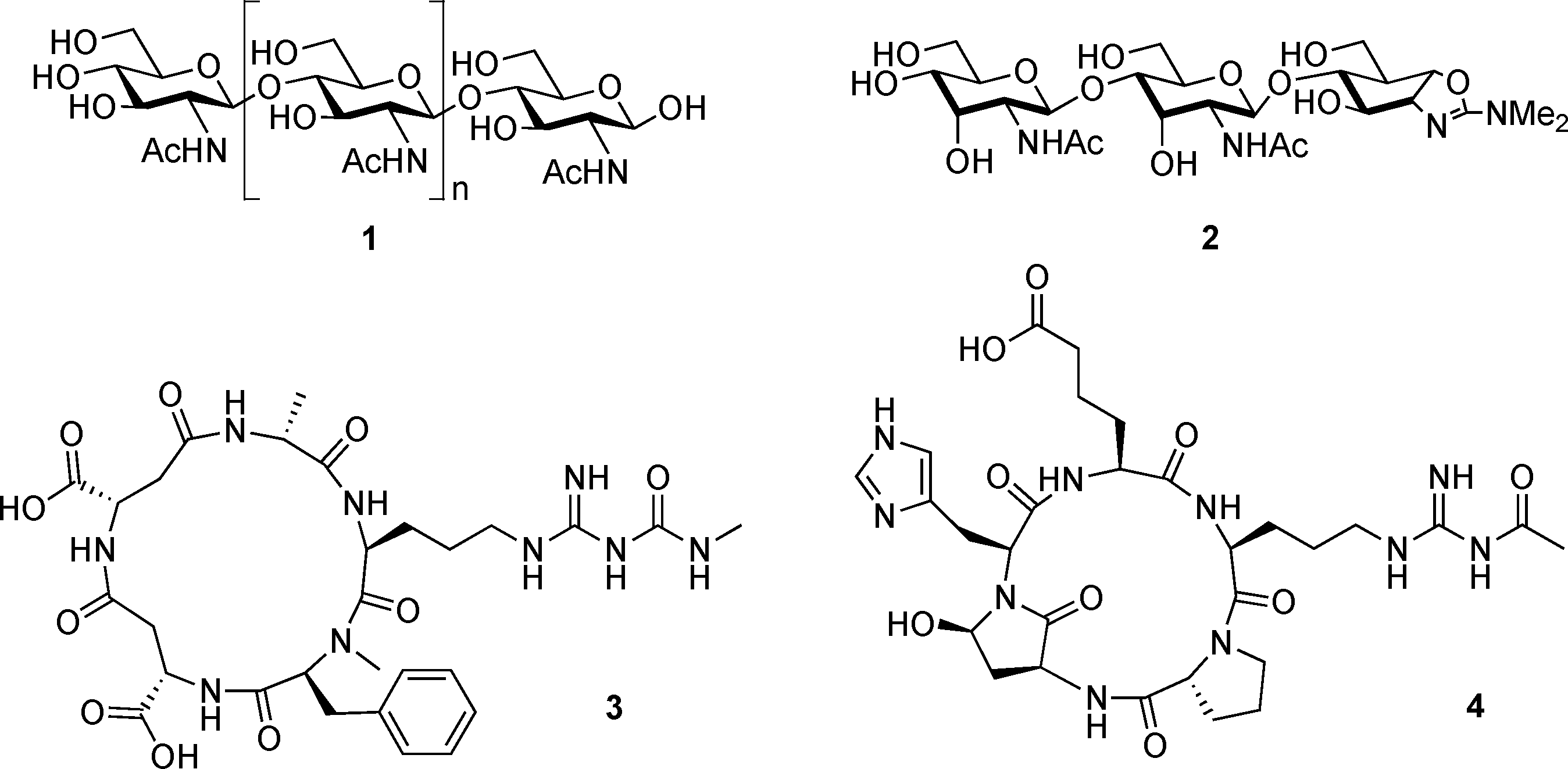
Chitin 1, allosamidin 2, argifin 3 and argadin 4.
CAS number: 104-15-4
Toluene-4-sulfonic acid is an arenesulfonic acid that is benzenesulfonic acid in which the hydrogen at position 4 is replaced by a methyl group. It is a member of toluenes and an arenesulfonic acid. It is a conjugate acid of a toluene-4-sulfonate.
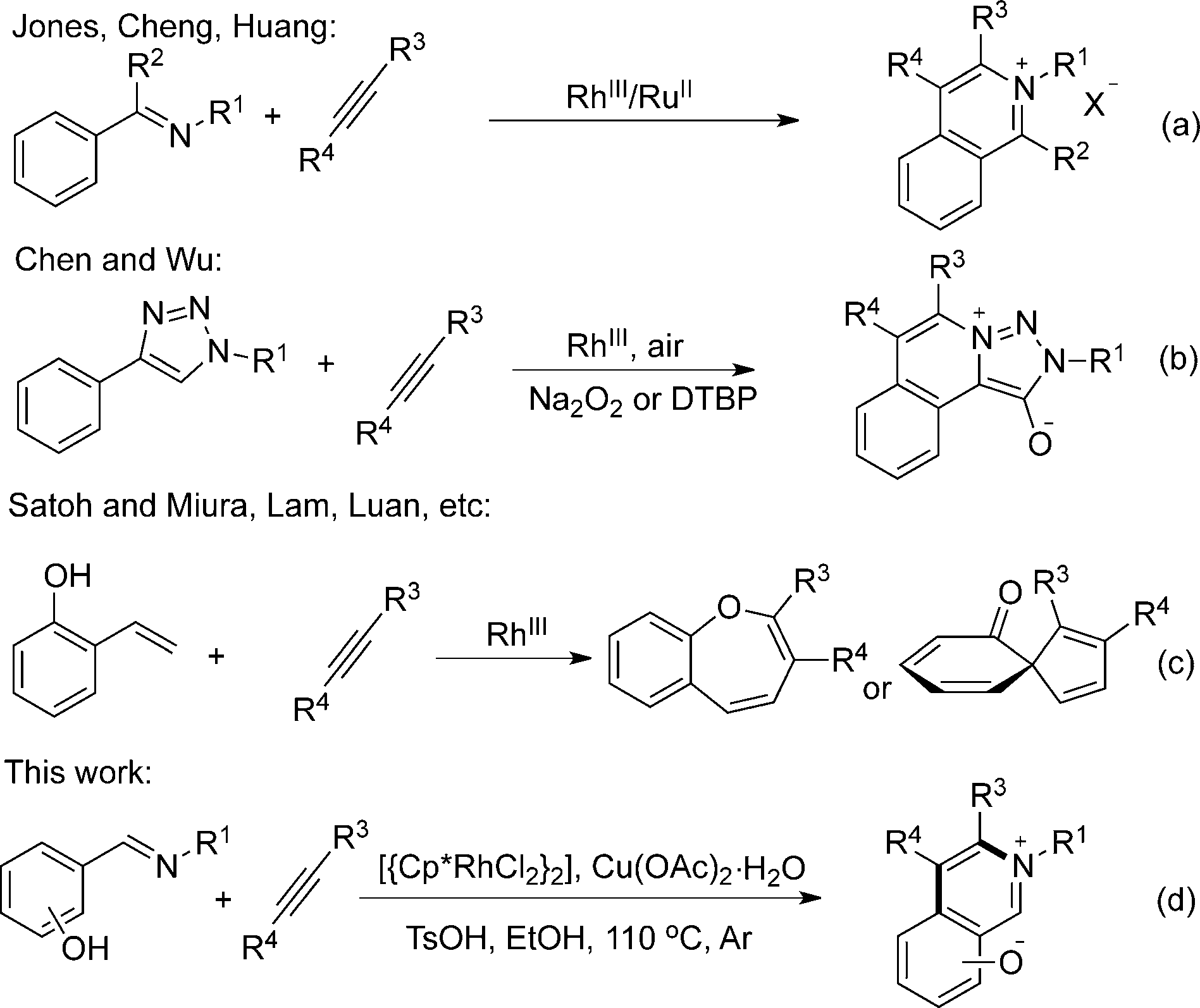
Transition-metal-catalyzed CÀH bond functionalizations.
CAS number: 104-92-7
4-bromoanisole is a monomethoxybenzene carrying a bromo substituent at position 4. It is a monomethoxybenzene and an organobromine compound.
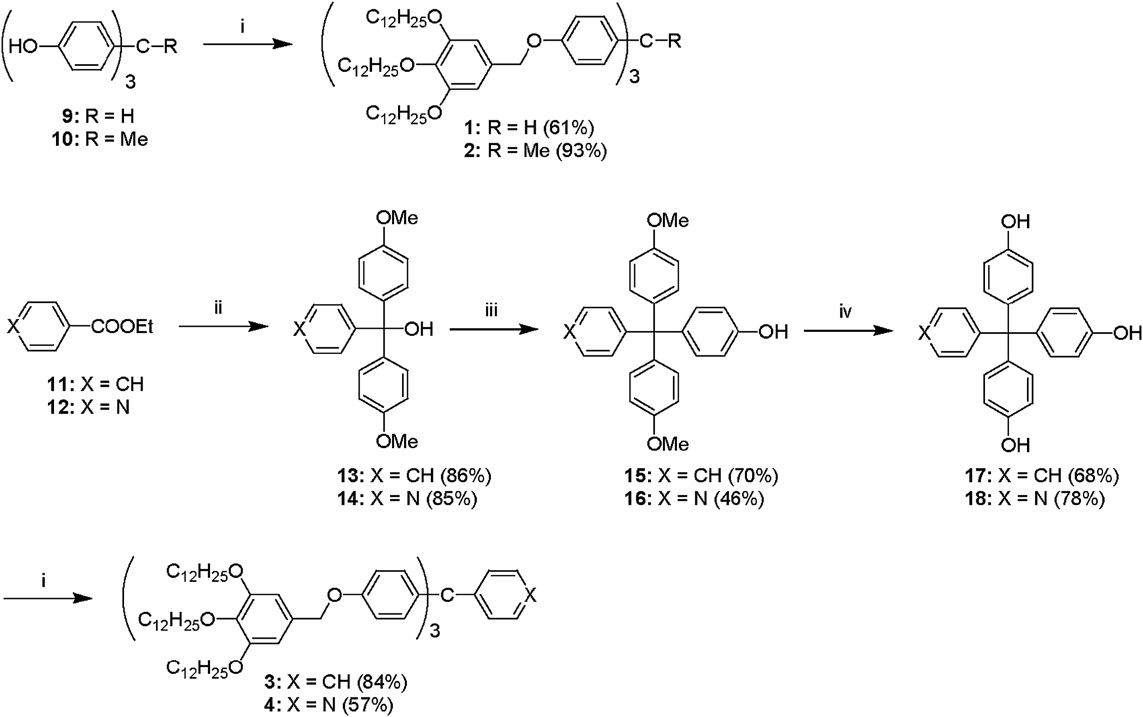
Synthesis of liquid crystals 1–4: (i) 3,4,5-tridodecyloxybenzyl chloride, K2CO3, DMF; (ii) 4-bromoanisole, Mg, THF; (iii) phenol, methanesulfonic acid; (iv) BBr3, CH2Cl2.
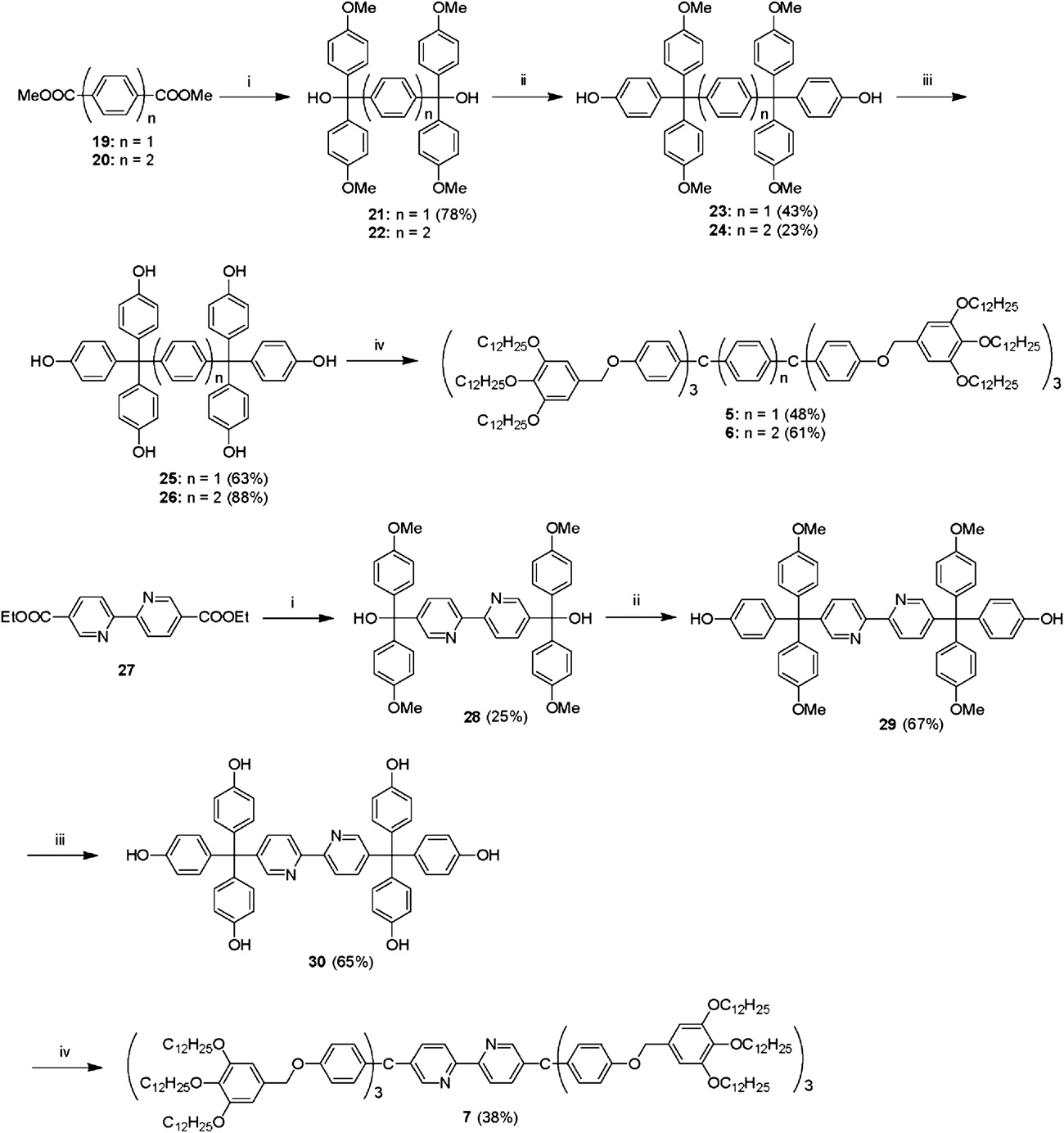
Synthesis of liquid crystals 5–7: (i) 4-bromoanisole, Mg, THF; (ii) phenol, methanesulfonic acid; (iii) BBr3, CH2Cl2; (iv) 3,4,5-tridodecyloxybenzyl chloride, K2CO3, DMF.
CAS number: 104-94-9
P-anisidine is a substituted aniline that is aniline in which the hydrogen para to the amino group has been replaced by a methoxy group. It is used as a reagent for the detection of oxidation products such as aldehydes and ketones in fats and oils. It has a role as a reagent and a genotoxin. It is a member of methoxybenzenes, a substituted aniline and a primary amino compound.
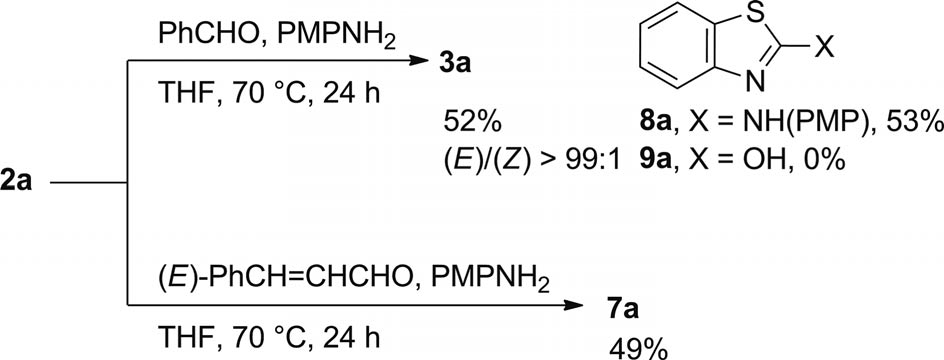
Reactions of sulfone 2a with aldehydes in the presence of p-methoxyaniline.
CAS number: 104053-06-7
5'-Acacccaattctgaaaatgg-3' is a single strand of DNA, also known as an oligonucleotide.
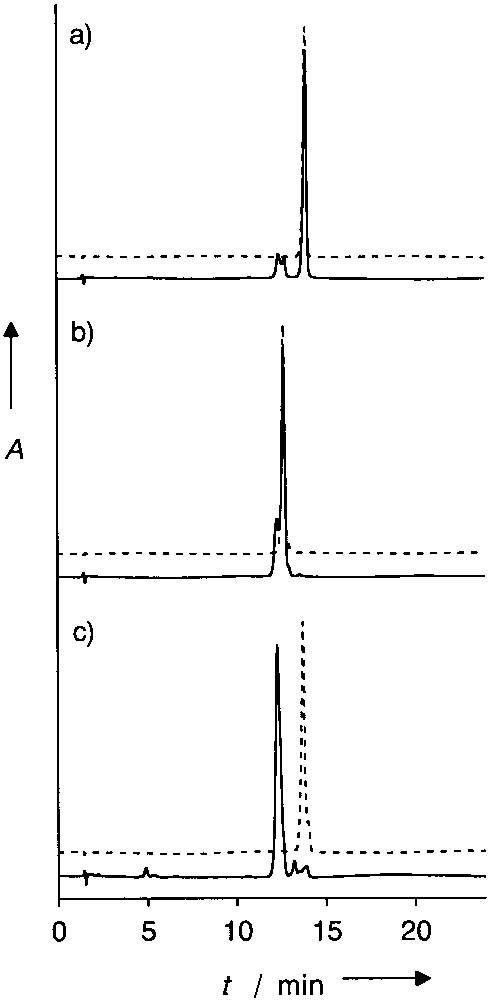
Ammonia treatment of the deprotected oligonucleotides.
CAS number: 10416-59-8
N,O-Bis(trimethylsilyl)acetamide (BSA) is a silylating agent used in organic chemistry, particularly for introducing trimethylsilyl (TMS) groups to molecules. It's a colorless, moisture-sensitive liquid, and a powerful reagent for protecting functional groups like amides, amines, alcohols, carboxylic acids, enols, and phenols. BSA is also used as a base precursor in certain reactions.
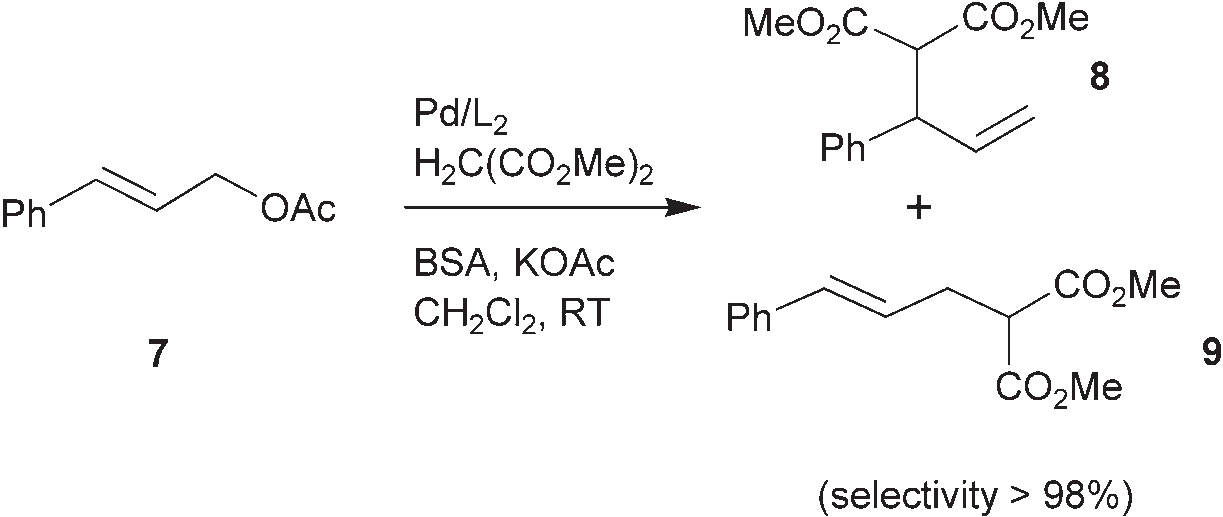
Palladium-catalyzed allylic substitution of the unsymmetrical substrate 7. BSA=N,O-Bis(trimethylsilyl)acetamide.
CAS number: 104352-01-4
Protonated arginine refers to the form of the amino acid arginine in which the guanidino group (–C(=NH)NH₂) is fully protonated, carrying a positive charge. This occurs under physiological pH (~7.4), where arginine's side chain remains positively charged due to its high pKa (~12.5).
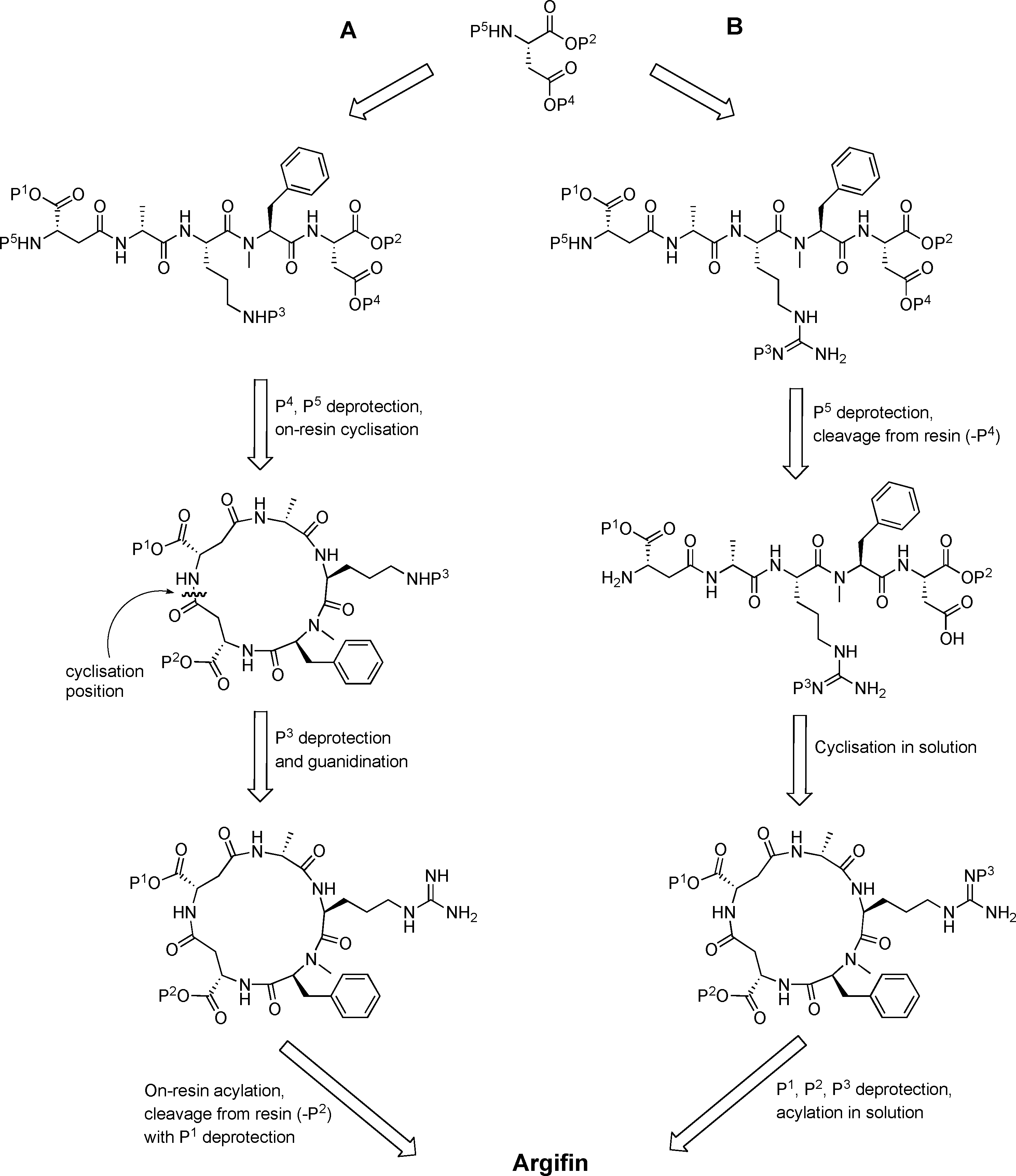
Synthetic approaches to argifin. Path A: All-solid-phase approach. Path B: Previous combined solid-phase–solution approach.