Chemicals list & Research Gallery
CAS number: 1092061-61-4
Crebinostat is a Novel Cognitive Enhancer that Inhibits Histone Deacetylase Activity and Modulates Chromatin-Mediated Neuroplasticity.
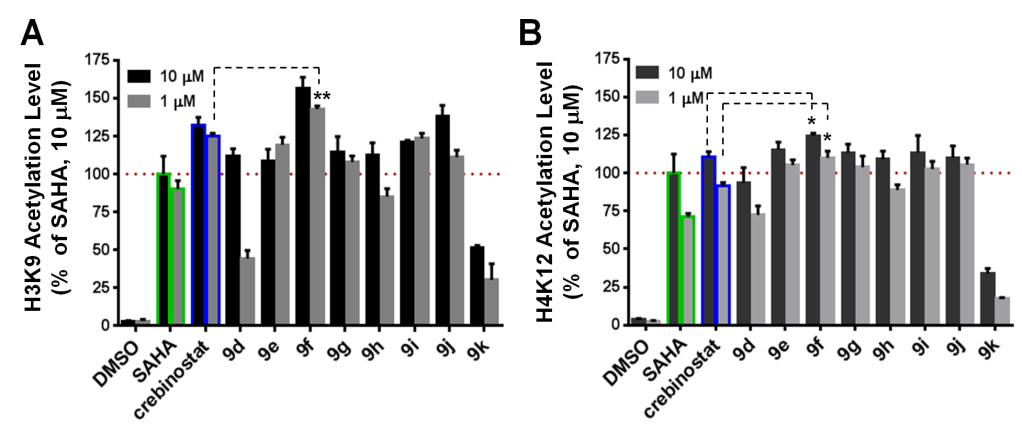
Crebinostat analogs induce histone H3K9 and H4K12 acetylation in mouse forebrain primary neuronal cultures.
CAS number: 109889-09-0
Granisetron is a Serotonin-3 Receptor Antagonist. The mechanism of action of granisetron is as a Serotonin 3 Receptor Antagonist.
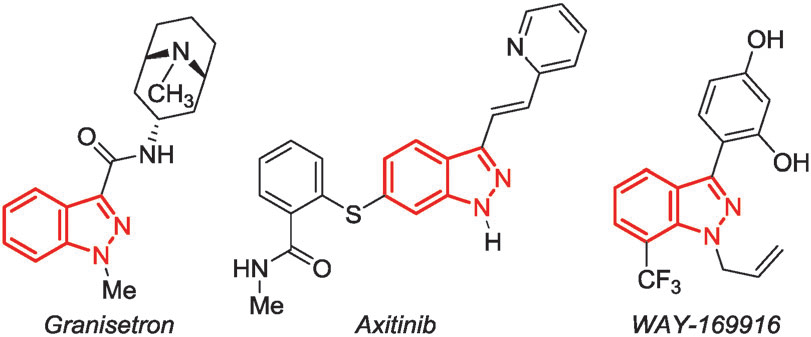
Some drugs and drug candidates containing the 1H-indazole structure.
CAS number: 110-00-9
Furan is a heterocyclic organic compound that consists of five aromatic rings that contain four carbon atoms and one oxygen.
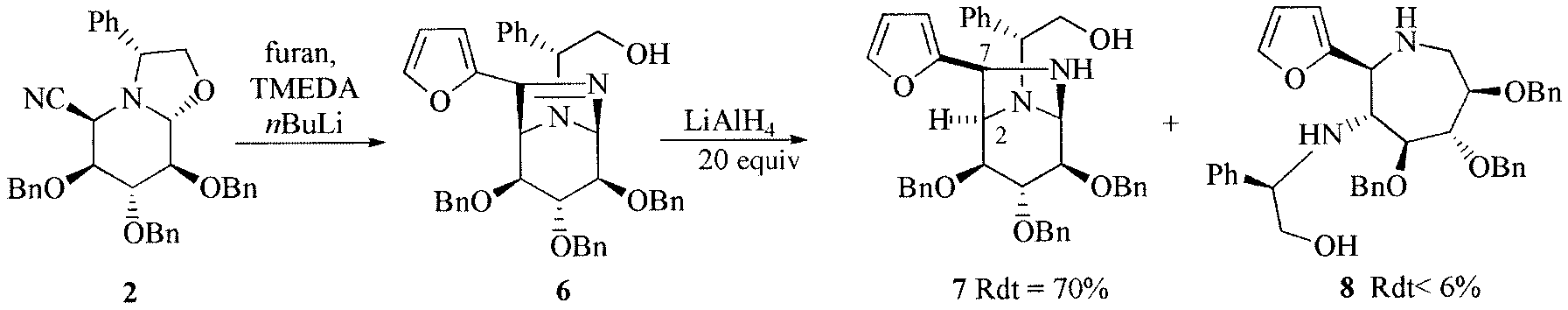
The use of furan as imine substituent.
CAS number: 110-02-1
Thiophene is a monocyclic heteroarene that is furan in which the oxygen atom is replaced by a sulfur. It has a role as a non-polar solvent.
![[4+2] and [4+4] cycloadditions of photogenerated azaxylylenes to the furan and thiophene pendant groups. 5’a denotes the minor hydroxy epimer in the thiophene [4+2] cycloadduct. Yields of the isolated products are shown.](http://www.wlxkc.cn/picture/3047941_02.png)
[4+2] and [4+4] cycloadditions of photogenerated azaxylylenes to the furan and thiophene pendant groups. 5’a denotes the minor hydroxy epimer in the thiophene [4+2] cycloadduct. Yields of the isolated products are shown.
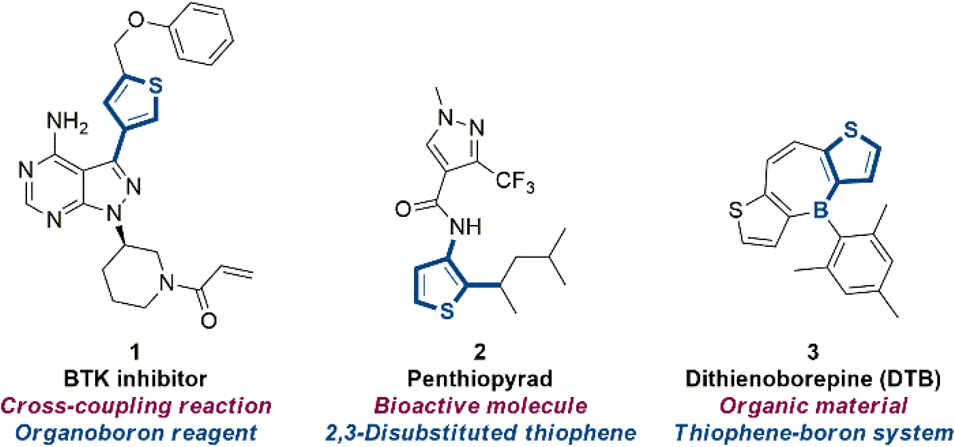
Synthetic importance of thiophene building blocks.
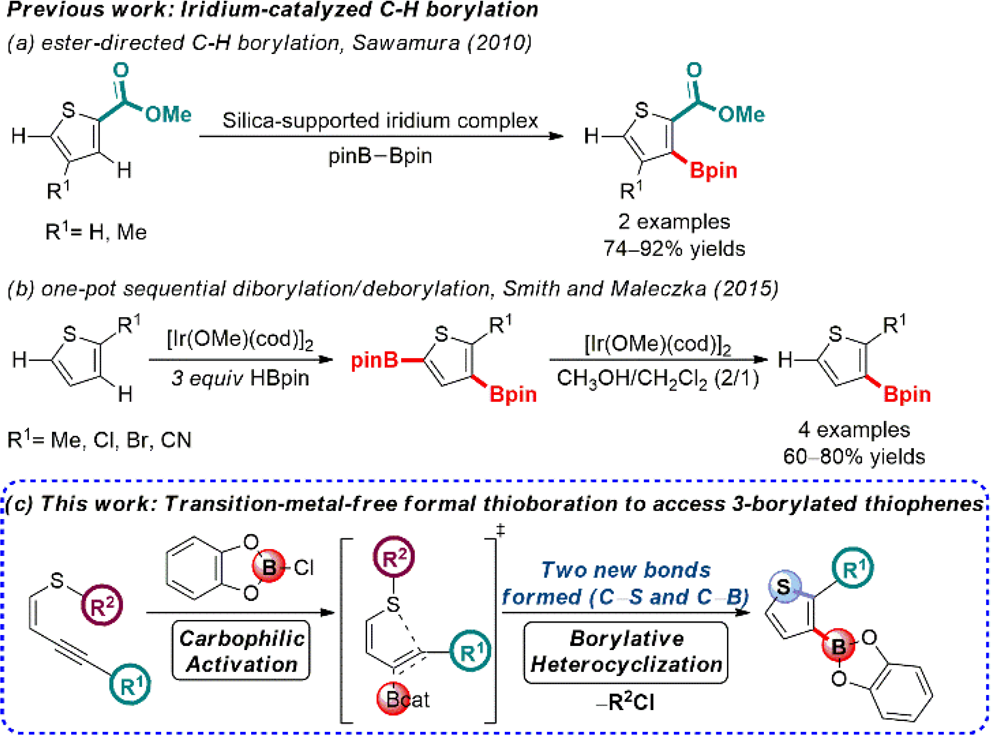
Current Strategies for the Borylation of Thiophenes
CAS number: 110-21-4
Biurea is a white powder.
![Synthesis of Azodicarboxamide [2]Rotaxanes 3 and 4 and Their Corresponding Hydrazodicarboxamide [2]Rotaxanes [2H]-3 and [2H]-4](http://www.wlxkc.cn/picture/5381702_03.png)
Synthesis of Azodicarboxamide [2]Rotaxanes 3 and 4 and Their Corresponding Hydrazodicarboxamide [2]Rotaxanes [2H]-3 and [2H]-4
CAS number: 110-52-1
1,4-Dibromobutane is a colorless to yellow liquid with a molecular weight of 215.91 g/mol. It is also known as tetramethylene dibromide. It is used as a reagent in various chemical reactions, particularly in the synthesis of polymers and other organic compounds.
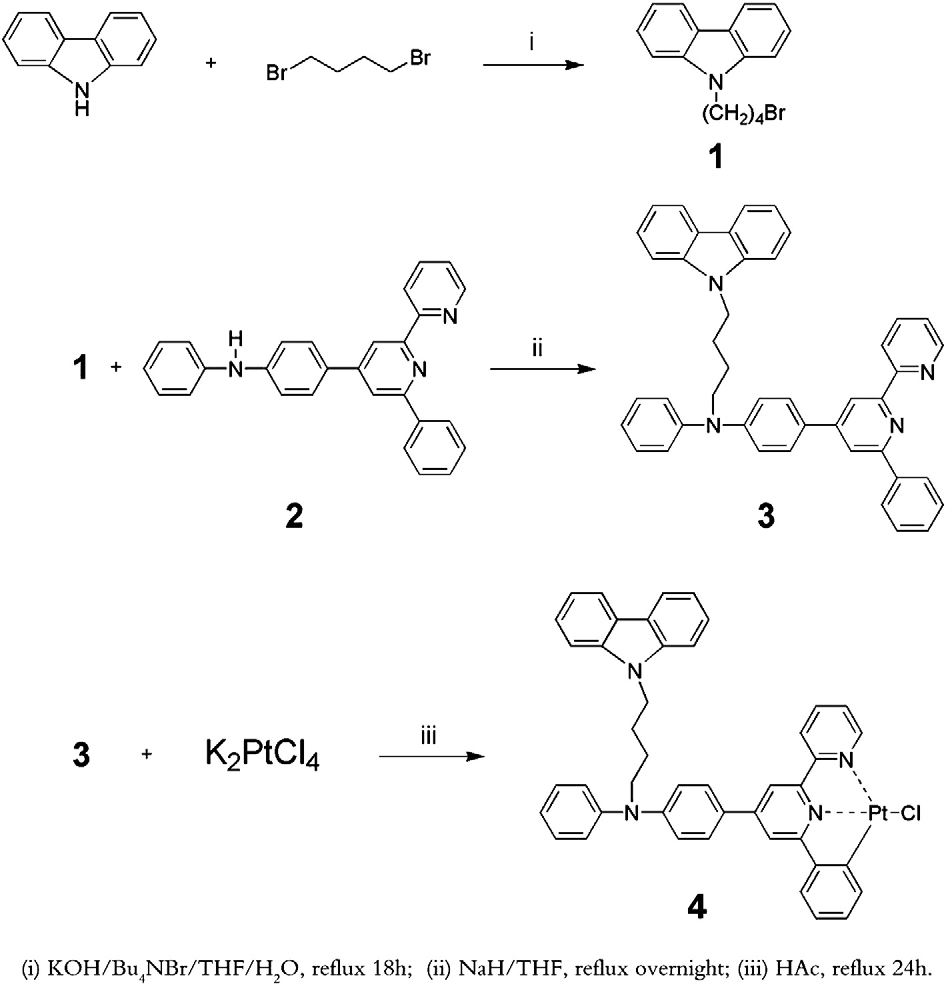
Carbazole was directly coupled with excess 1,4-dibromobutane under strong alkaline conditions to give 9-(4-bromobutane)carbazole (1). 4-(p-bromophenyl)-6-phenyl-2,2′-bipyridine was synthesized by the Kröhnke method. A strong palladium-catalyzed Buchwald method and an efficient Pd(OAc)2/DPEphos catalyst/ligand system were used to promote the condensation of aniline with 4-(p-bromophenyl)-6-phenyl-2,2′-bipyridine. The resulting intermediate (2) was then reacted with 9-(4-bromobutane)carbazole (1) to give the ligand HL (3). The ligand HL (3) and K2PtCl4 were refluxed in glacial acetic acid for 24 h to synthesize the cyclometalated Pt(II) chloride 4 with a yield of more than 80%.
CAS number: 110-54-3
n-Hexane is a chemical made from crude oil. Pure n-Hexane is a colorless liquid with a slightly disagreeable odor. It is highly flammable, and its vapors can be explosive. Puren-Hexane is used in laboratories. n-Hexane can cause male reproductive toxicity according to an independent committee of scientific and health experts.
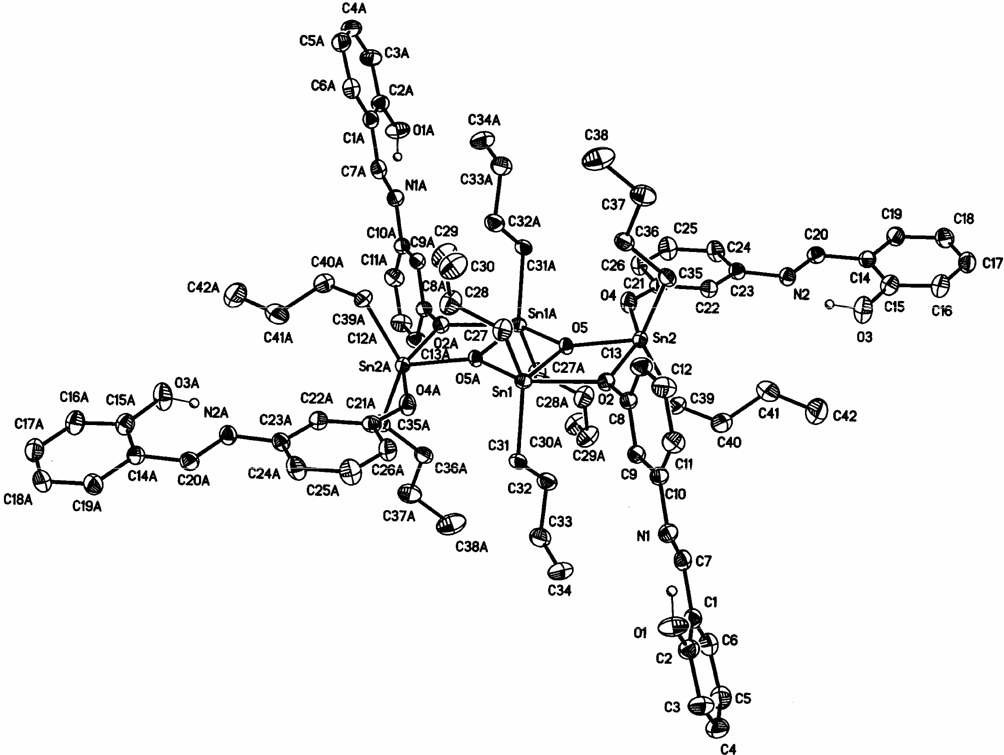
Complex 2.2 was crystallized from hexane. The ORTEP view is shown in Figure 2.
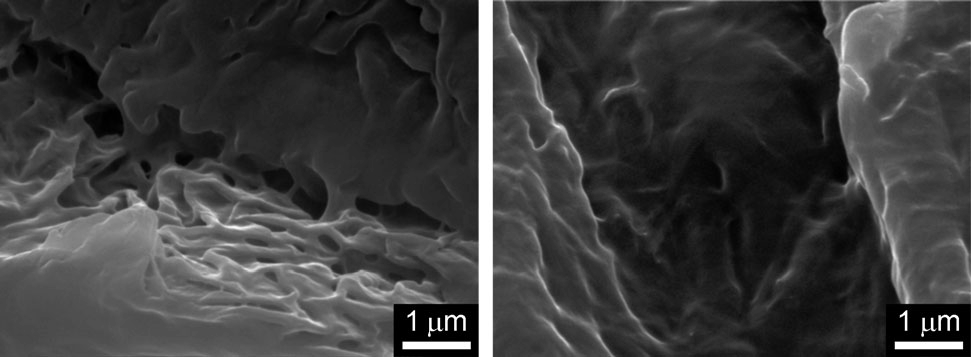
Scanning electron micrographs (SEM): a) dried gel of 1a from hexane; b) dried gel of 2b from hexane.
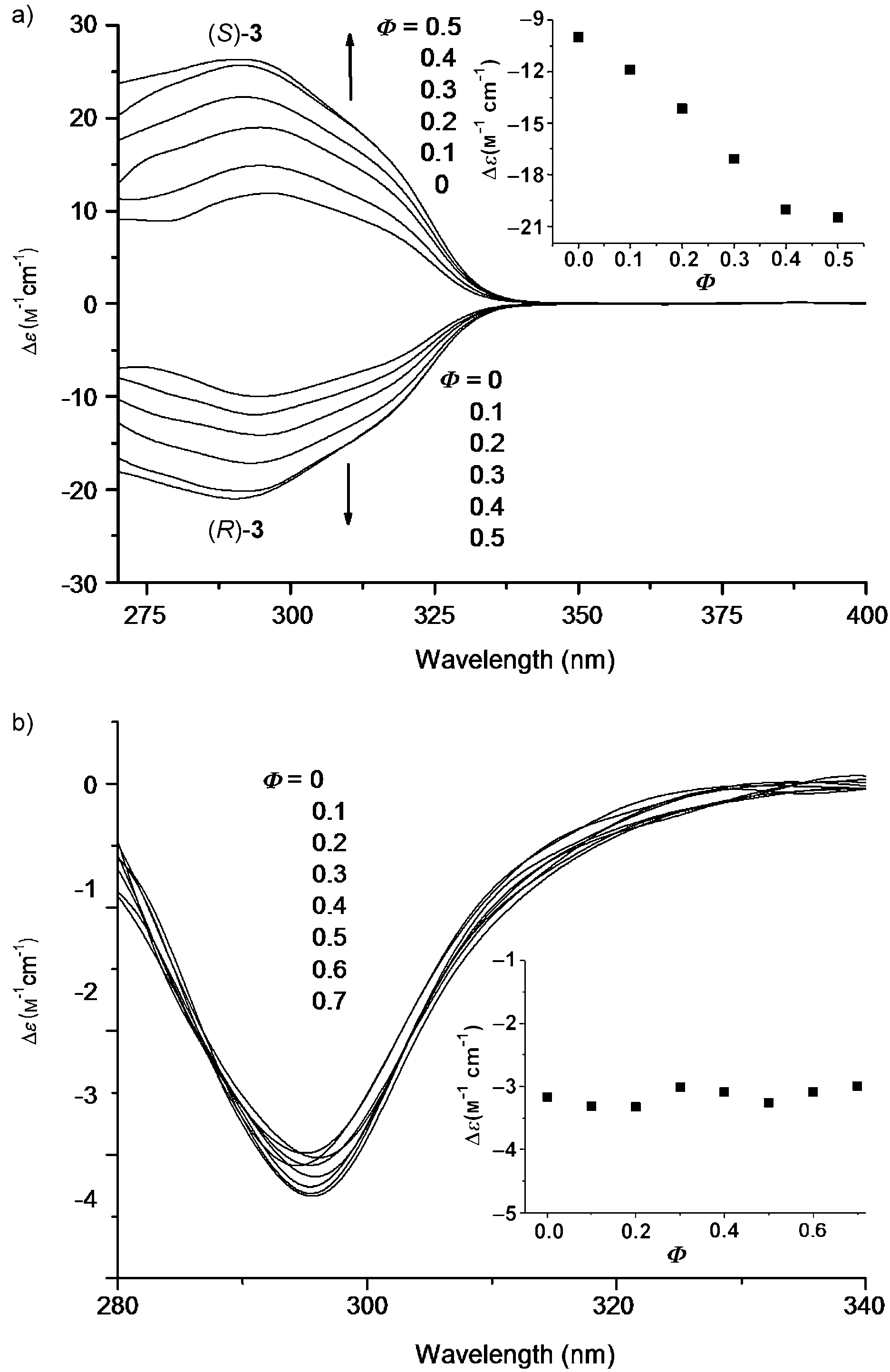
CD spectra of a) enantiomers (R)-3 and (S)-3 and b) (R)-4c in chloroform/n-hexane mixture at 25 °C.
CAS number: 110-71-4
1,2-dimethoxyethane is a diether that is the 1,2-dimethyl ether of ethane-1,2-diol. It has a role as a non-polar solvent. It is functionally related to an ethylene glycol.
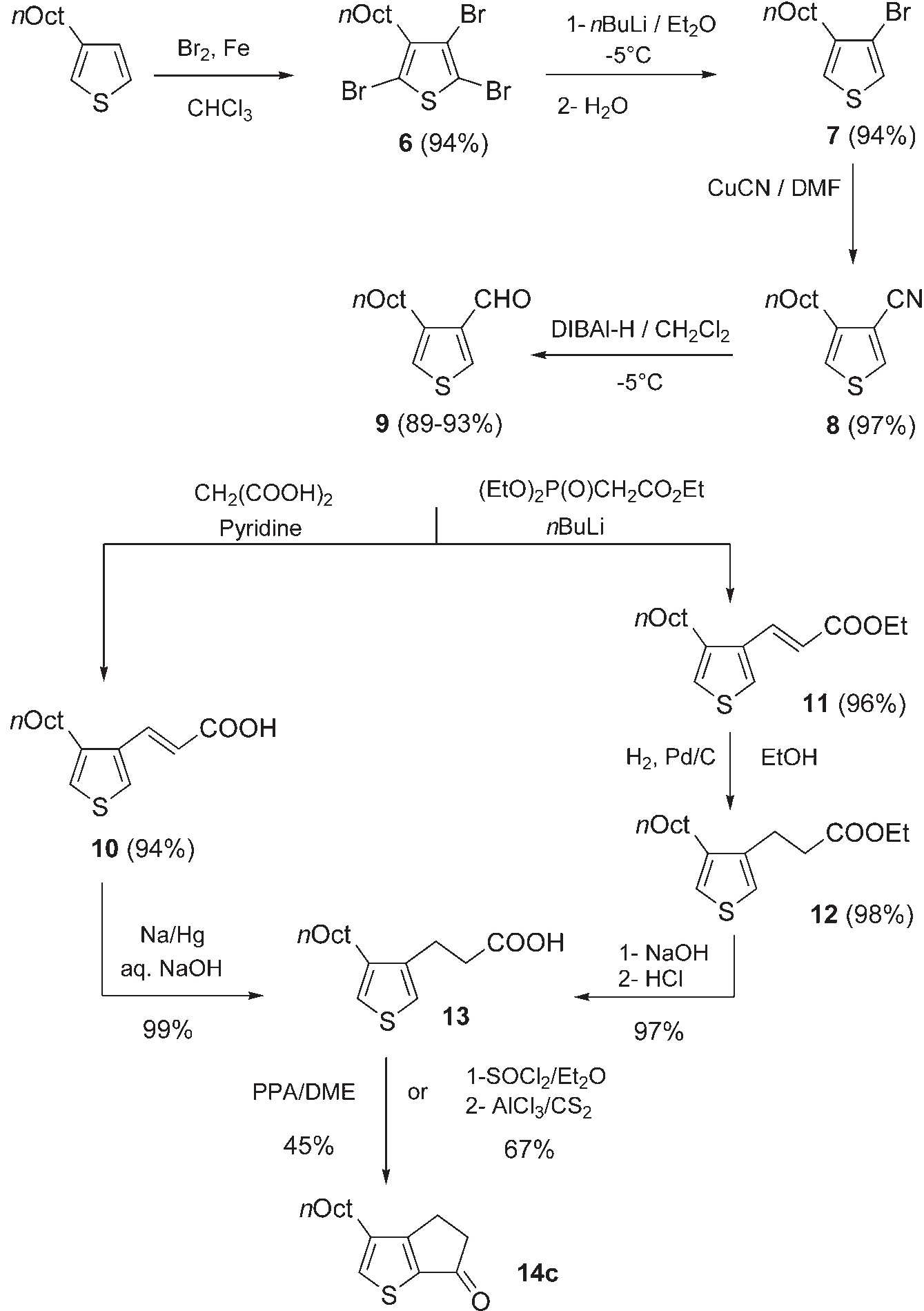
Synthesis of cyclic ketone 14c. Oct =octyl, DmF=N,N-di-methylformamide, DIBAl-H=diisobutylaluminum hydride, PPA=poly-phosphoric acid, DmE=dimethoxyethane.
CAS number: 110-82-7
Cyclohexane is an alicyclic hydrocarbon comprising a ring of six carbon atoms; the cyclic form of hexane, used as a raw material in the manufacture of nylon.
![Plots of the fluorescence quantum yields determined in THF/H2O solutions by using 9,10-diphenylanthracene (F=90% in cyclohexane) as the internal standard versus the fraction of water in the solvent. Insets: photo- graphs of the TPE–Ar luminogens in the THF/water mixture (fw =99%) taken under the illumination of a 365 nm UV lamp. Excitation wavelengths [nm]: 320 for TPE–ptol and TPE–mtol, and 310 for the other six fluorophores.](http://www.wlxkc.cn/picture/3386517_12.png)
Plots of the fluorescence quantum yields determined in THF/H2O solutions by using 9,10-diphenylanthracene (F=90% in cyclohexane) as the internal standard versus the fraction of water in the solvent. Insets: photo- graphs of the TPE–Ar luminogens in the THF/water mixture (fw =99%) taken under the illumination of a 365 nm UV lamp. Excitation wavelengths [nm]: 320 for TPE–ptol and TPE–mtol, and 310 for the other six fluorophores.
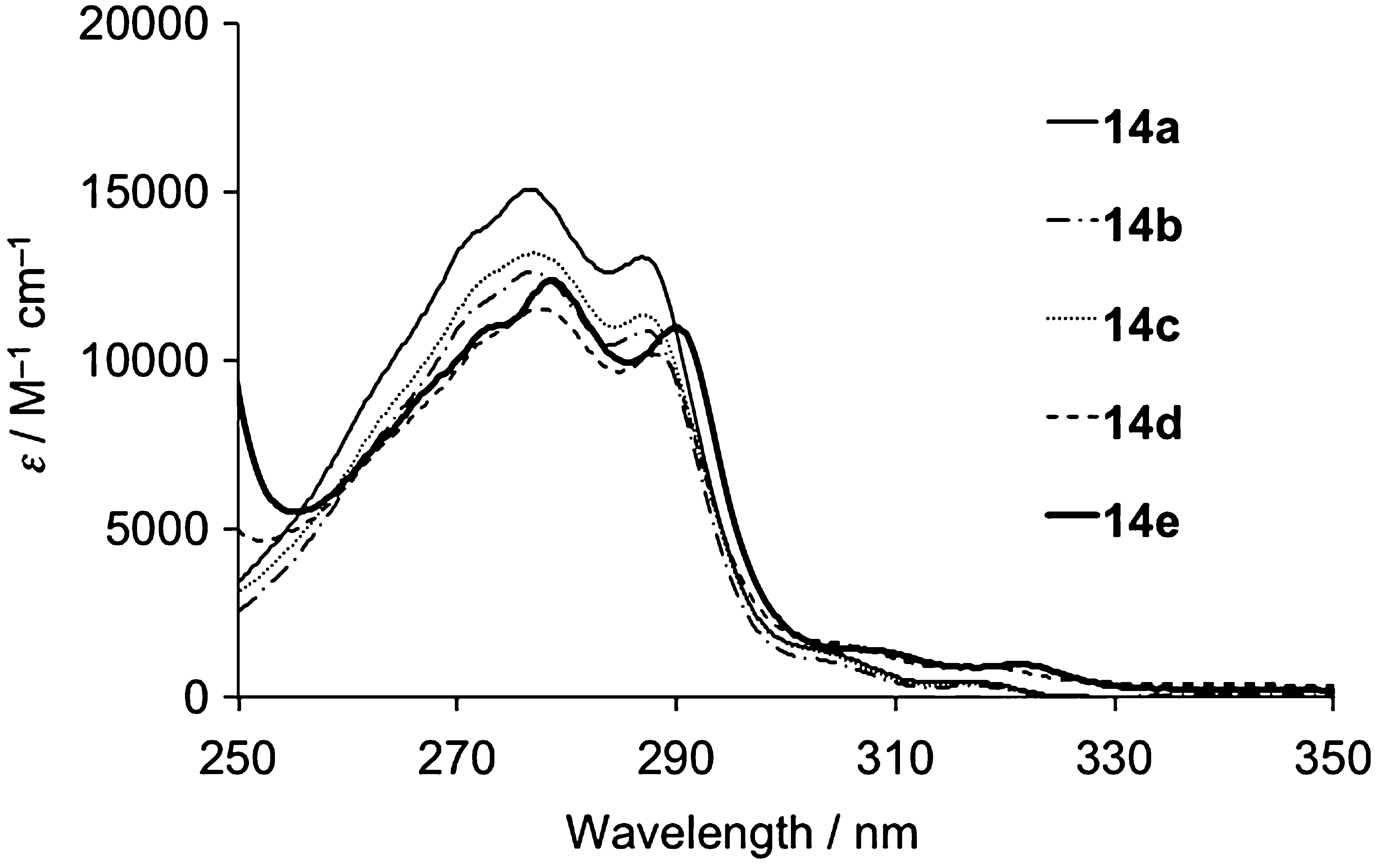
Absorption spectra of 14 in cyclohexane.
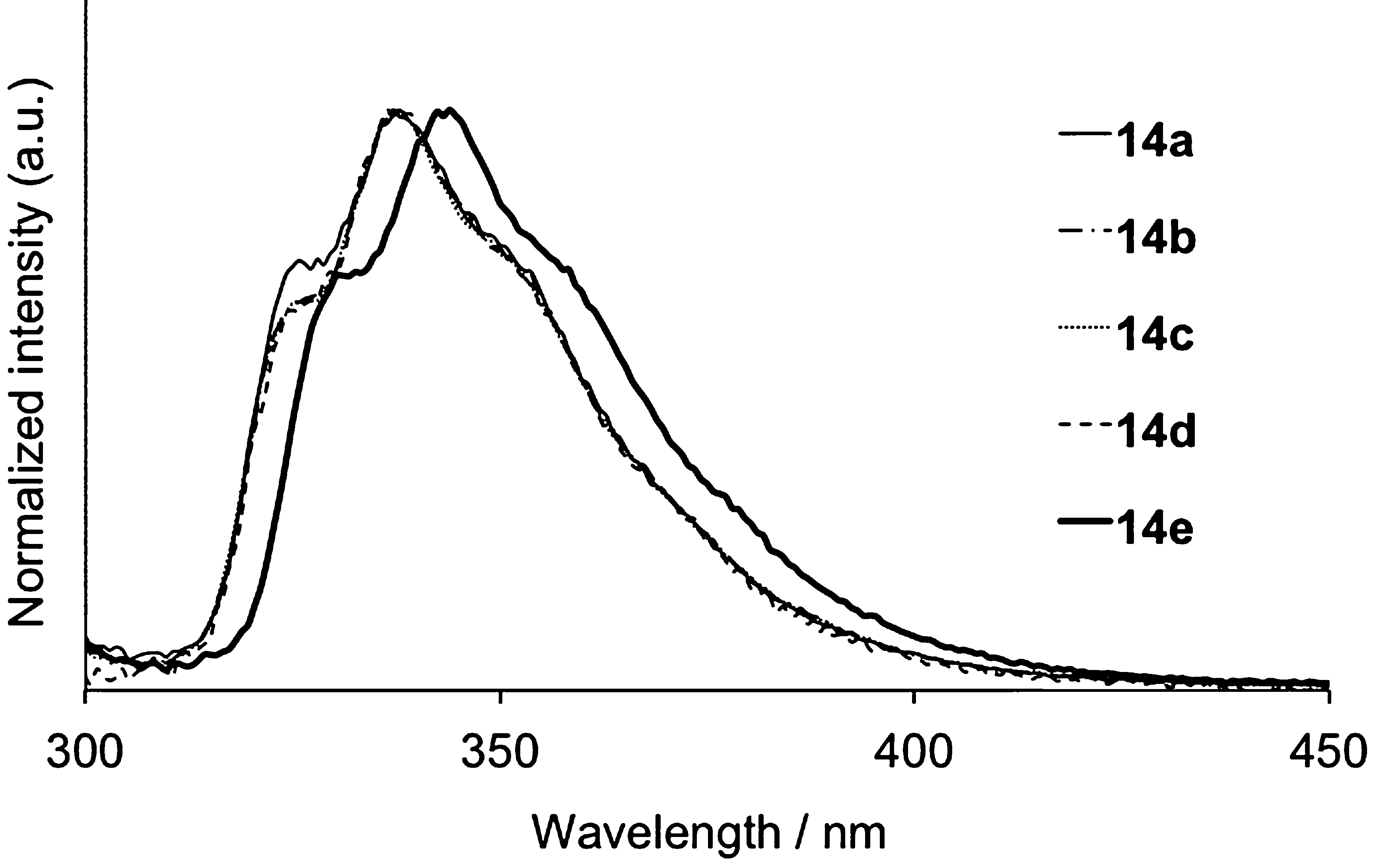
Fluorescence spectra of 14 in cyclohexane.
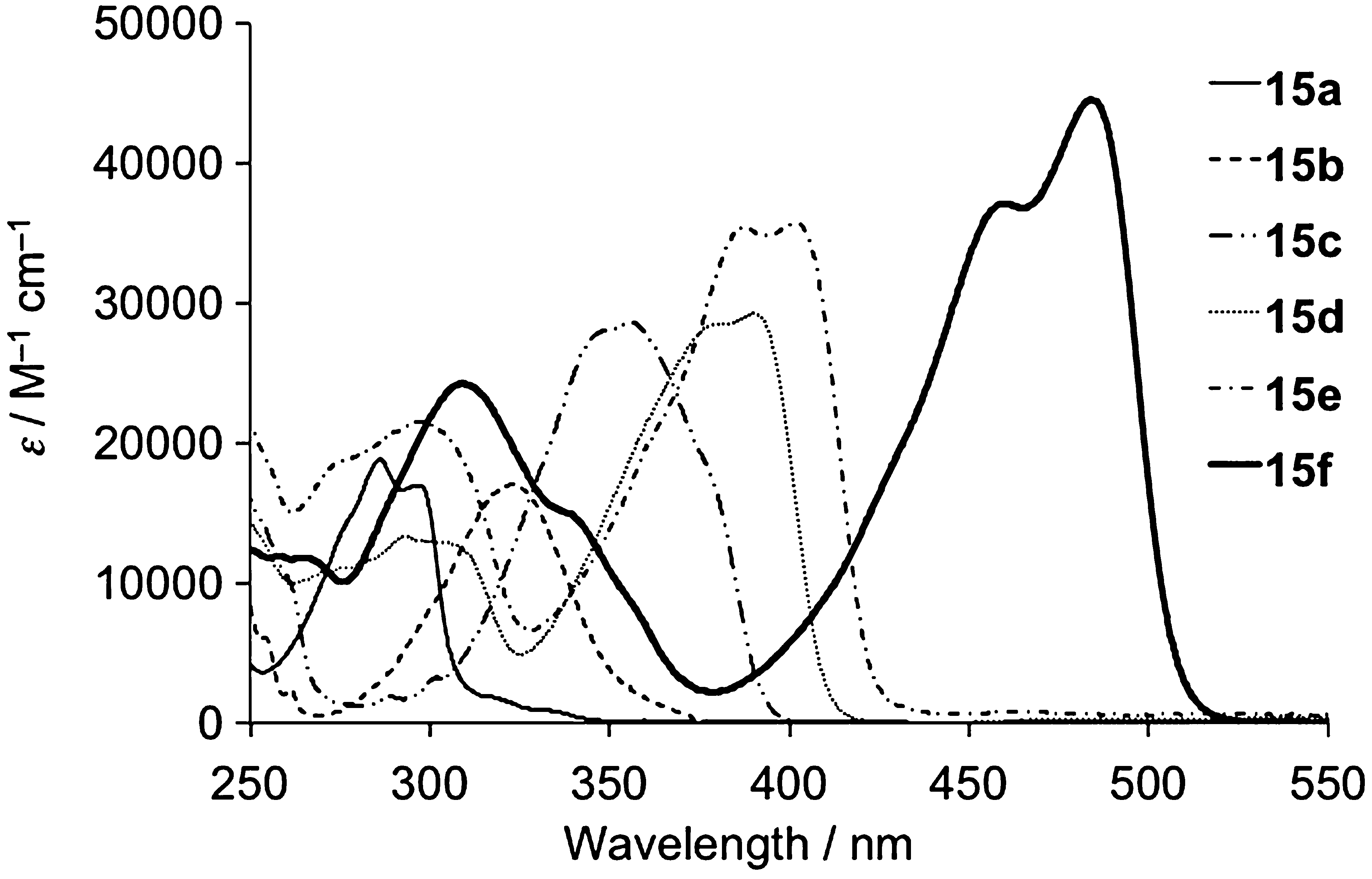
Absorption spectra of 15 in cyclohexane.
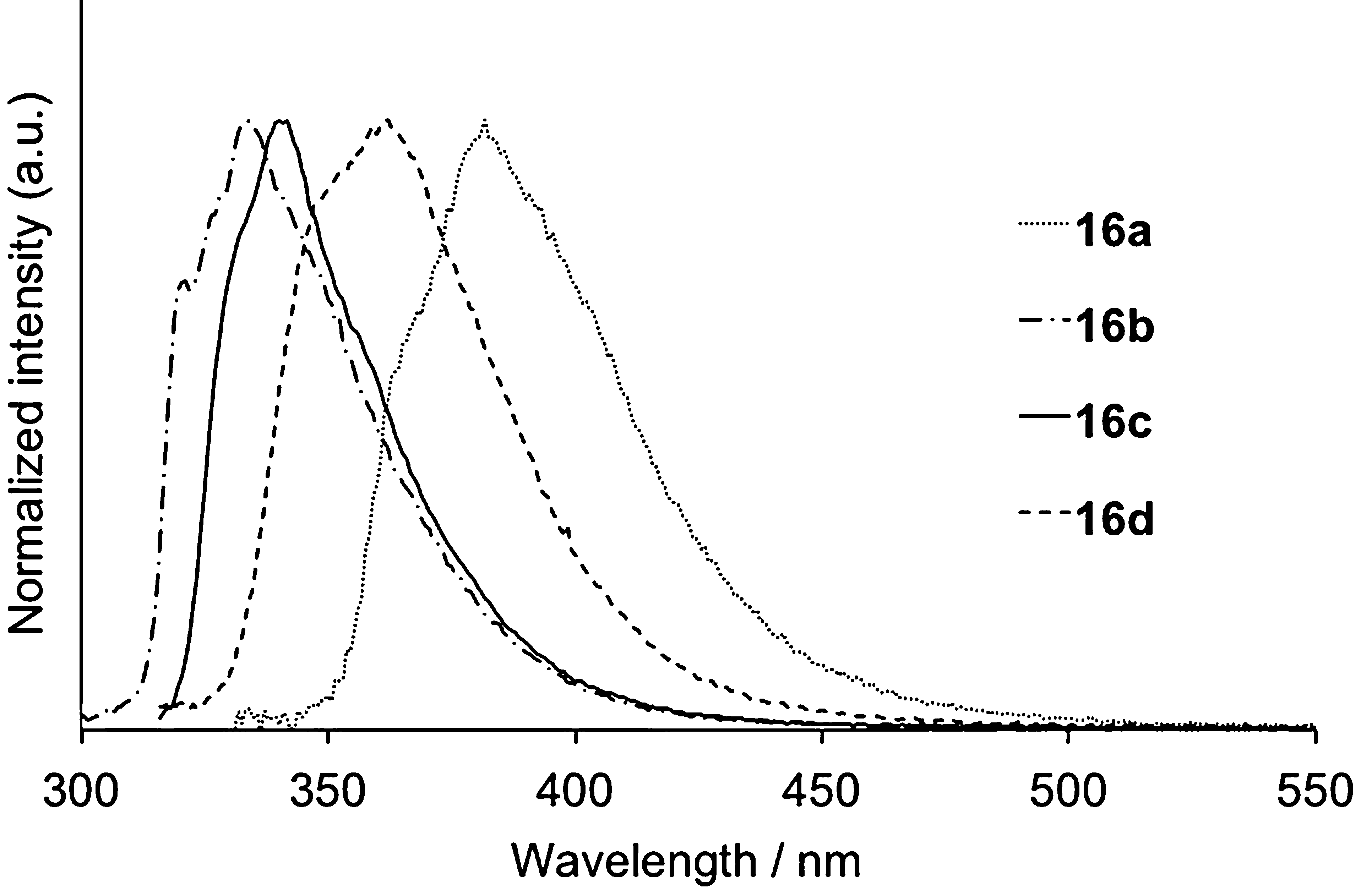
Fluorescence spectra of 16 in cyclohexane.
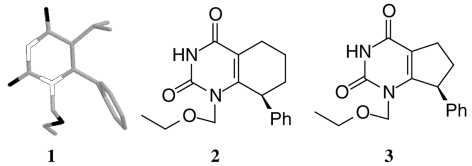
1 Crystal structure of MKC-442 when bound to RT. 2 Structure of cyclohexane-annelated MKC-442 analogue. 3 Structure of cyclopentane-annelated MKC-442 analogue.
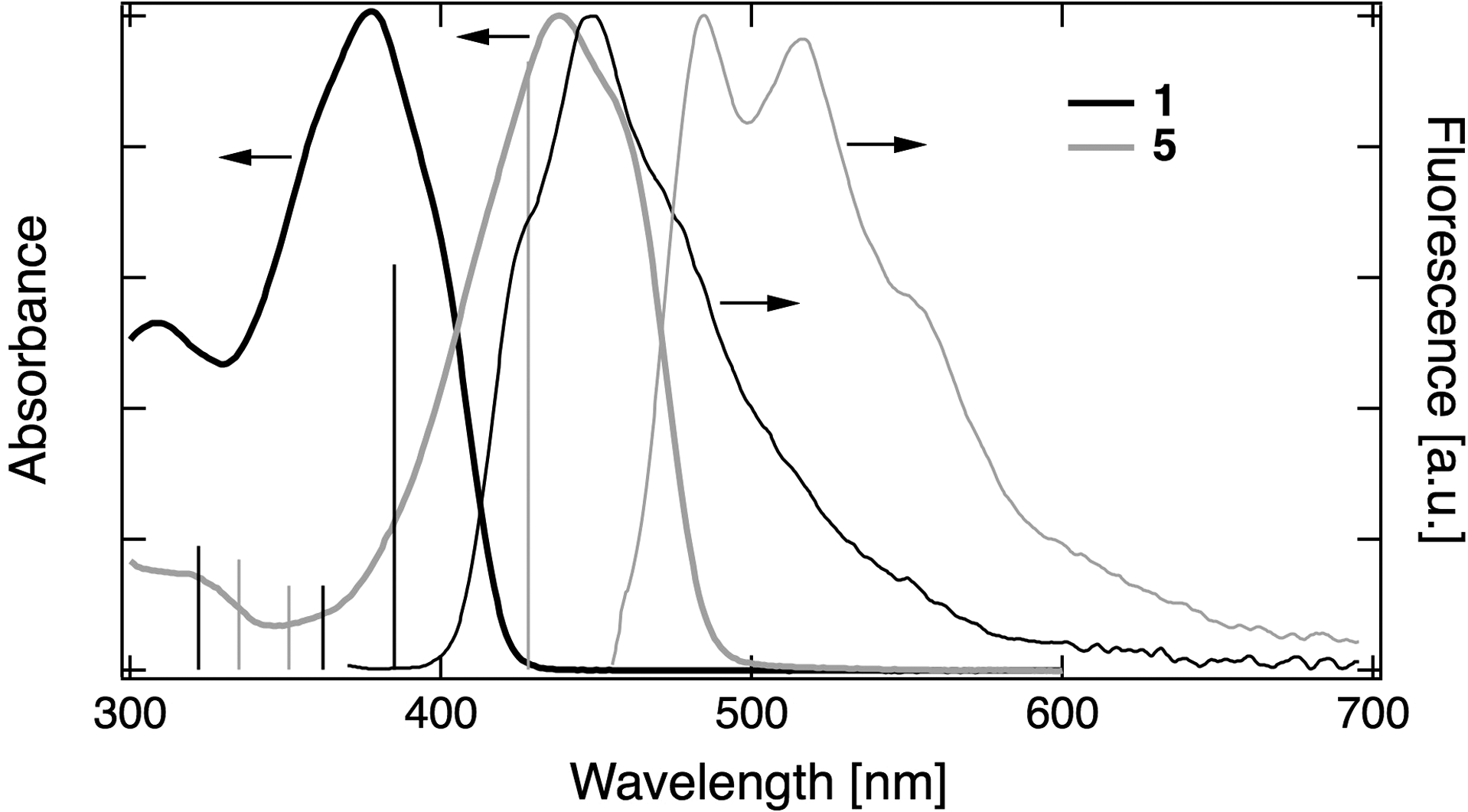
Absorption and fluorescence spectra of 1 and 5 in cyclohexane at 300 K.
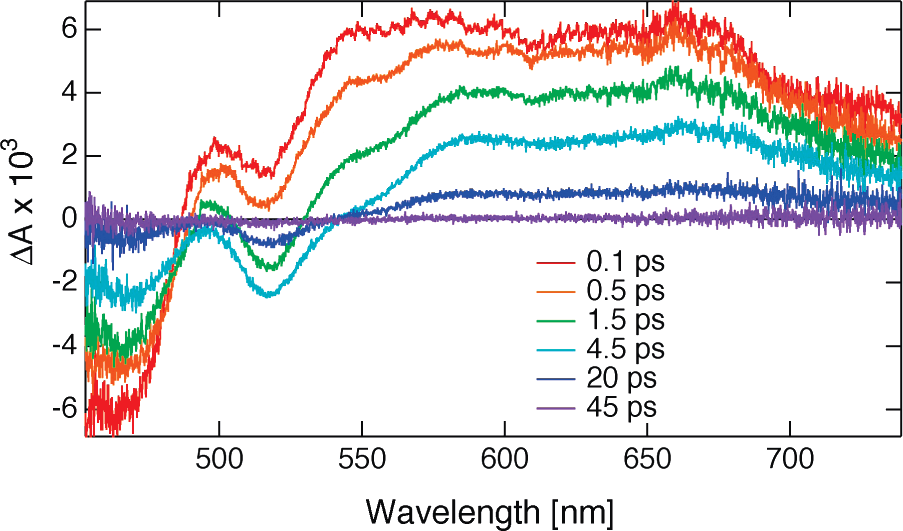
TA spectra recorded with 5 in cyclohexane at different time delays after excitation.
CAS number: 110-85-0
Piperazine is an azacycloalkane that consists of a six-membered ring containing two nitrogen atoms at opposite positions. It has a role as an anthelminthic drug. It is a saturated organic heteromonocyclic parent, an azacycloalkane and a member of piperazines. It is a conjugate base of a piperazinium(2+).
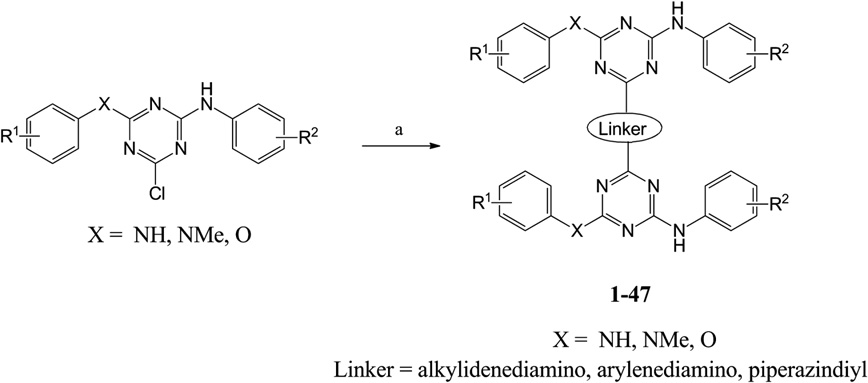
Synthesis of triazine dimers 1–47. Reagents and conditions: (a) diaminoalkanes/diaminobenzene/piperazine, DIPEA, dry dioxane, 110 °C.