Chemicals list & Research Gallery
CAS number: 130306-02-4
Tezacitabine is a synthetic pyrimidine nucleoside analogue with potential antineoplastic activity. Phosphorylated by cellular kinases, tezacitabine is converted into its active diphosphate and triphosphate metabolites. Tezacitabine diphosphate binds to and irreversibly inhibits the activity of the enzyme ribonucleotide reductase (RNR), which may result in the inhibition of DNA synthesis in tumor cells and tumor cell apoptosis.
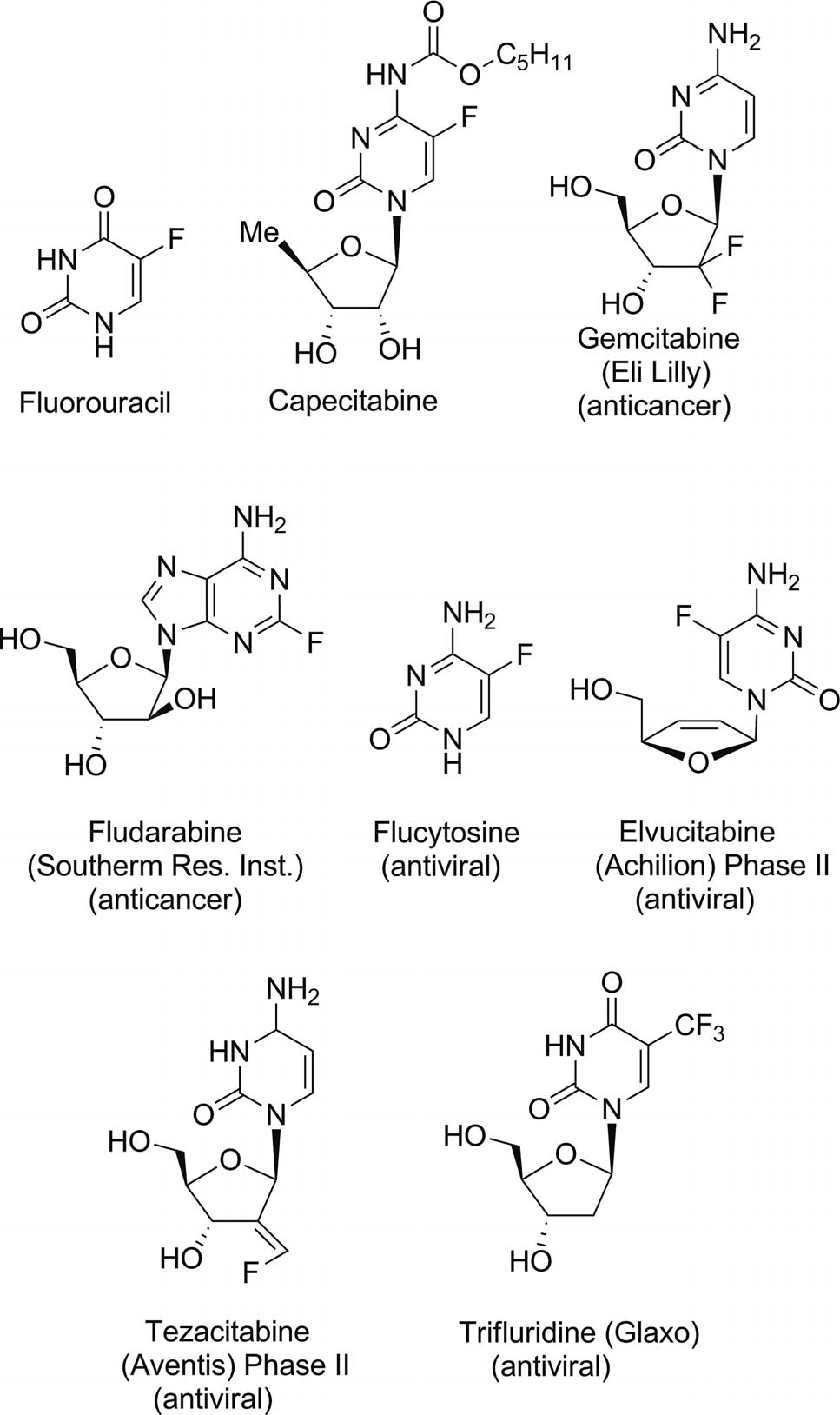
Fluorinated purine and pyrimidine antimetabolites clinically used for the treatment of numerous cancers and viral infections: Fluorouracil, capecitabine, gemcitabine, fludarabine, flucytosine, elvucitabine, tezacitabine, trifluridine.
CAS number: 13039-56-0
5-Deoxy-L-arabinose is a chemical compound, specifically a pentose sugar. It is an analog of L-arabinose, lacking a hydroxyl group at the fifth carbon (C5). It's known for being a strong inhibitor of β-galactosidase.
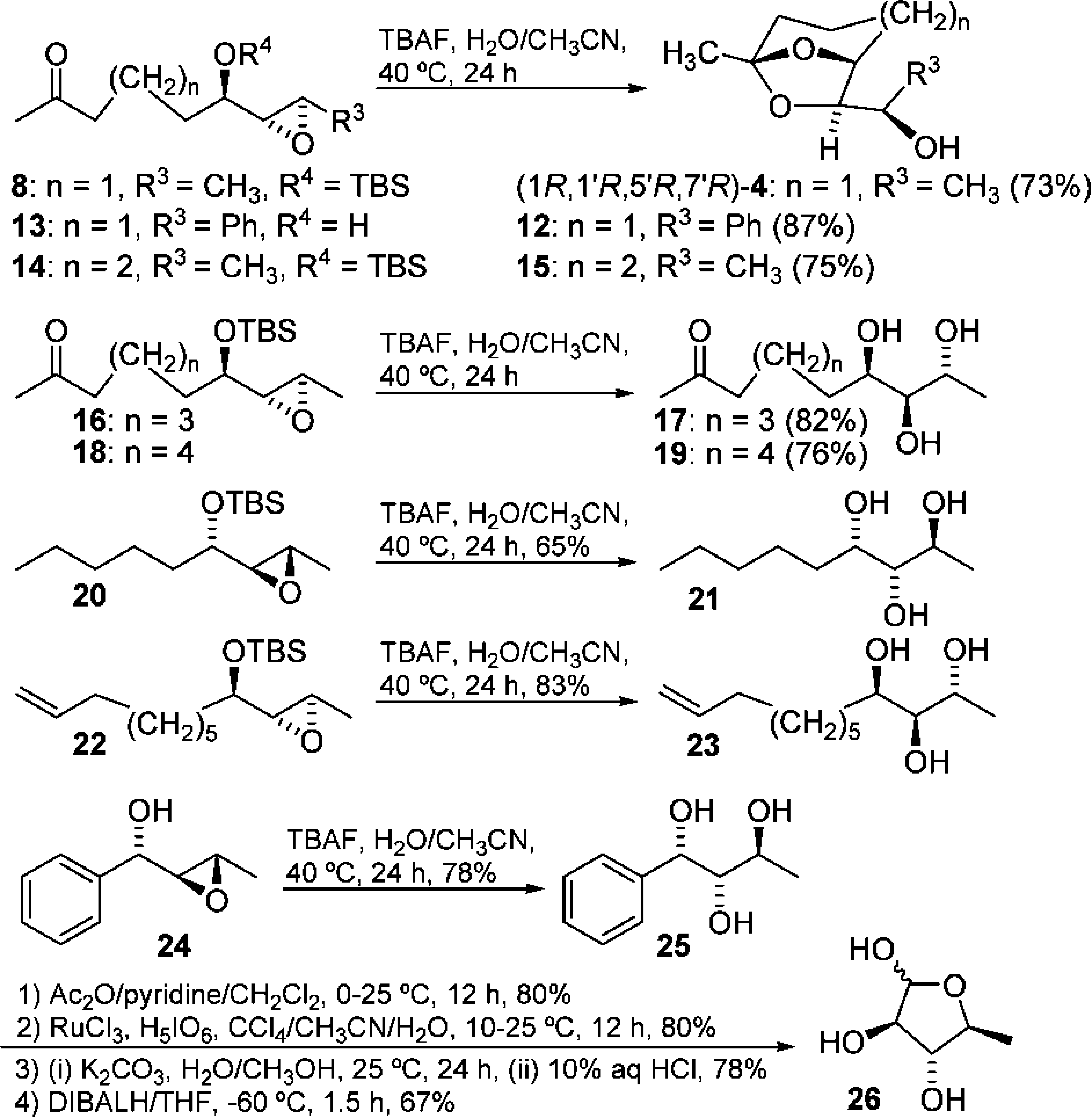
TBAF-Promoted Hydrolysis of a Variety of Epoxyalcohols and Synthesis of 5-Deoxy-L-arabinose
CAS number: 131-48-6
N-acetyl-beta-neuraminic acid is n-Acetylneuraminic acid with beta configuration at the anomeric centre. It has a role as an epitope. It is functionally related to a beta-neuraminic acid. It is a conjugate acid of a N-acetyl-beta-neuraminate.
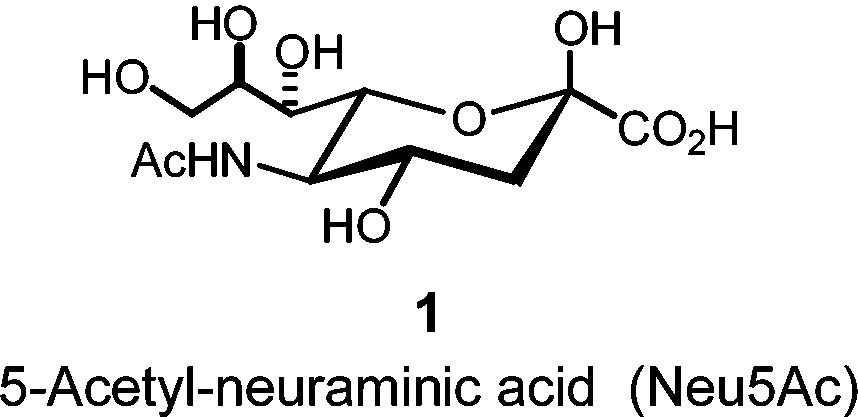
The most common sialic acid found in Nature: N-acetylneuraminic acid.
CAS number: 1310-65-2
Lithium hydroxide, solution appears as a clear to water-white liquid which may have a pungent odor. Contact may cause severe irritation to skin, eyes, and mucous membranes. It may be toxic by ingestion, inhalation and skin absorption. It is used to make other chemicals.
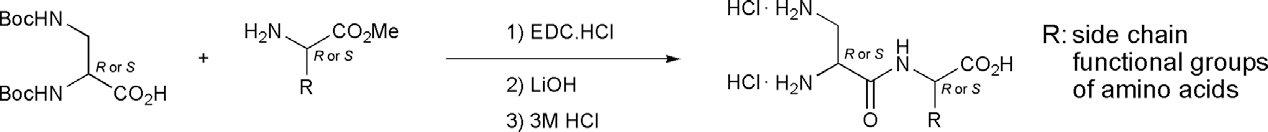
Synthesis of Dap dipeptides.
CAS number: 13149-00-3
Cis-hexahydrophthalic anhydride is a chemical compound, specifically a cyclic anhydride, derived from hexahydrophthalic acid. It is predominantly the cis isomer, meaning the two carboxyl groups are on the same side of the cyclohexane ring. It is used as a reactant in various chemical syntheses and as a component in epoxy resin curing agents and other applications.
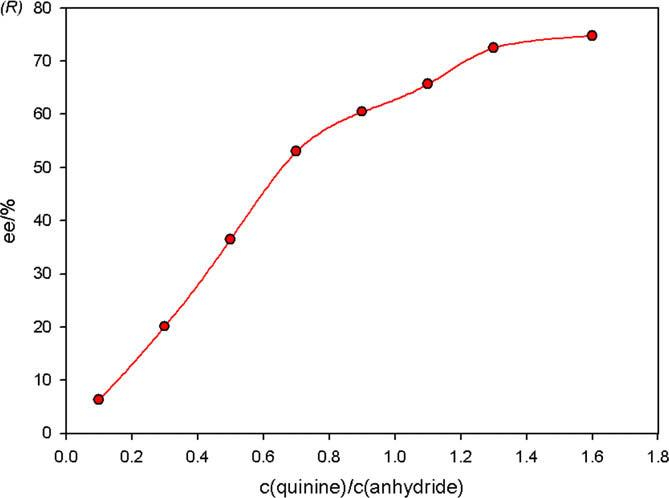
Influence of quinine loading on the desymmetrization of cis-1,2-Cyclohexanedicarboxylic anhydride 7.
CAS number: 131543-22-1
WIN 55212-2 is a organic heterotricyclic compound that is 5-methyl-3-(morpholin-4-ylmethyl)-2,3-dihydro[1,4]oxazino[2,3,4-hi]indole substituted at position 6 by a 1-naphthylcarbonyl group. It has a role as an analgesic, a neuroprotective agent and an apoptosis inhibitor. It is an organic heterotricyclic compound, a member of morpholines, a naphthyl ketone and a synthetic cannabinoid.
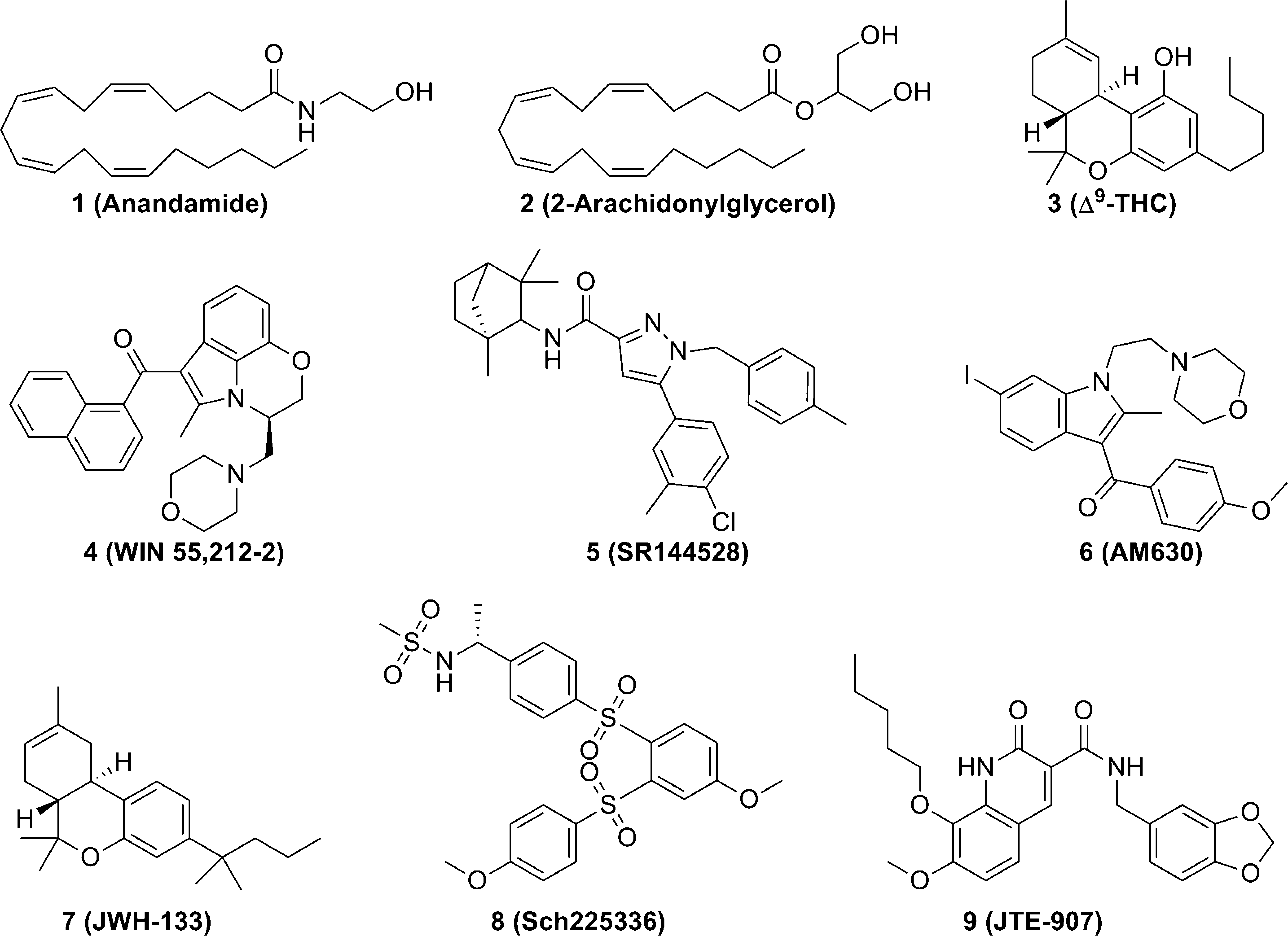
Representative cannabinoids with various chemical scaffolds: Anandamide, 2-Arachidonylglycerol, Win-55212-2, SR 144528, JWH-133, JTE-907.
CAS number: 1317-61-9
Ferriferrous Oxide, also known as Iron (II,III) oxide (Fe3O4), is a black ore of IRON that forms opaque crystals and exerts strong magnetism.
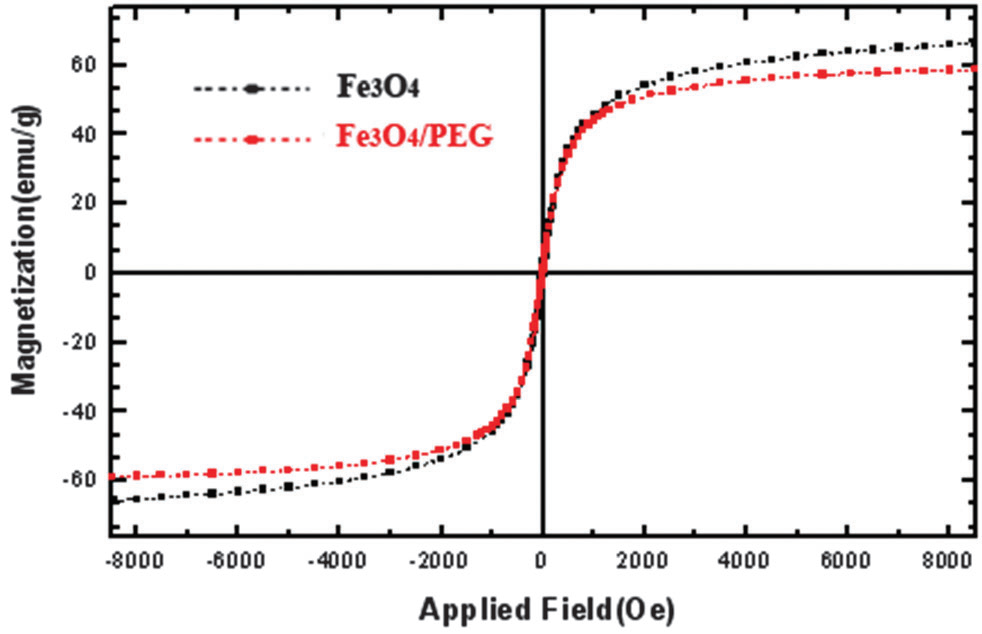
Magnetization curves obtained by VSM at room temperature of Fe3O4 MNPs and Fe3O4 MNPs coated by PEG.
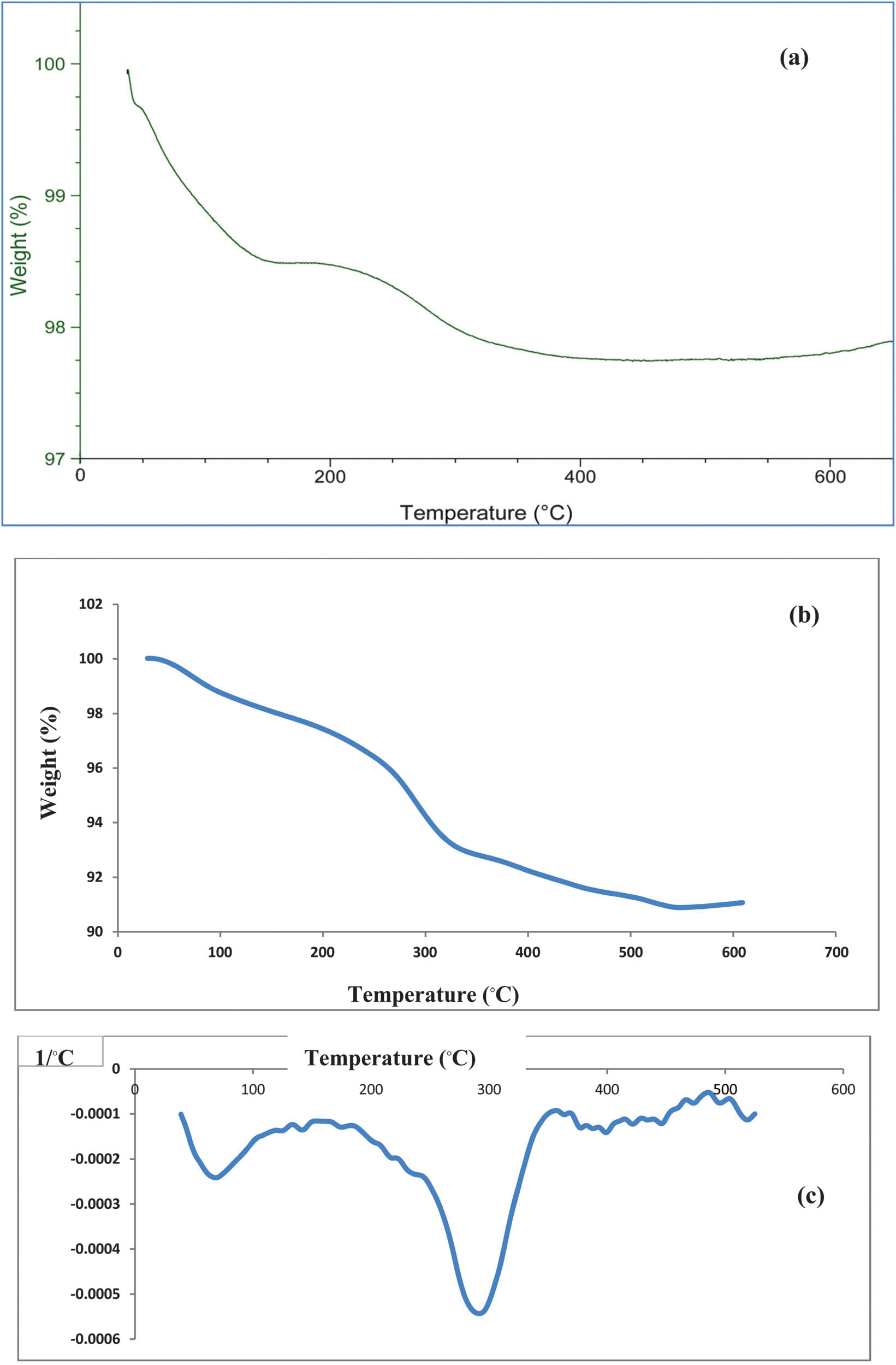
TGA curves of (a) Fe3O4, (b) Fe3O4–PEG and (c) DTA curve of Fe3O4–PEG composite nanoparticles.
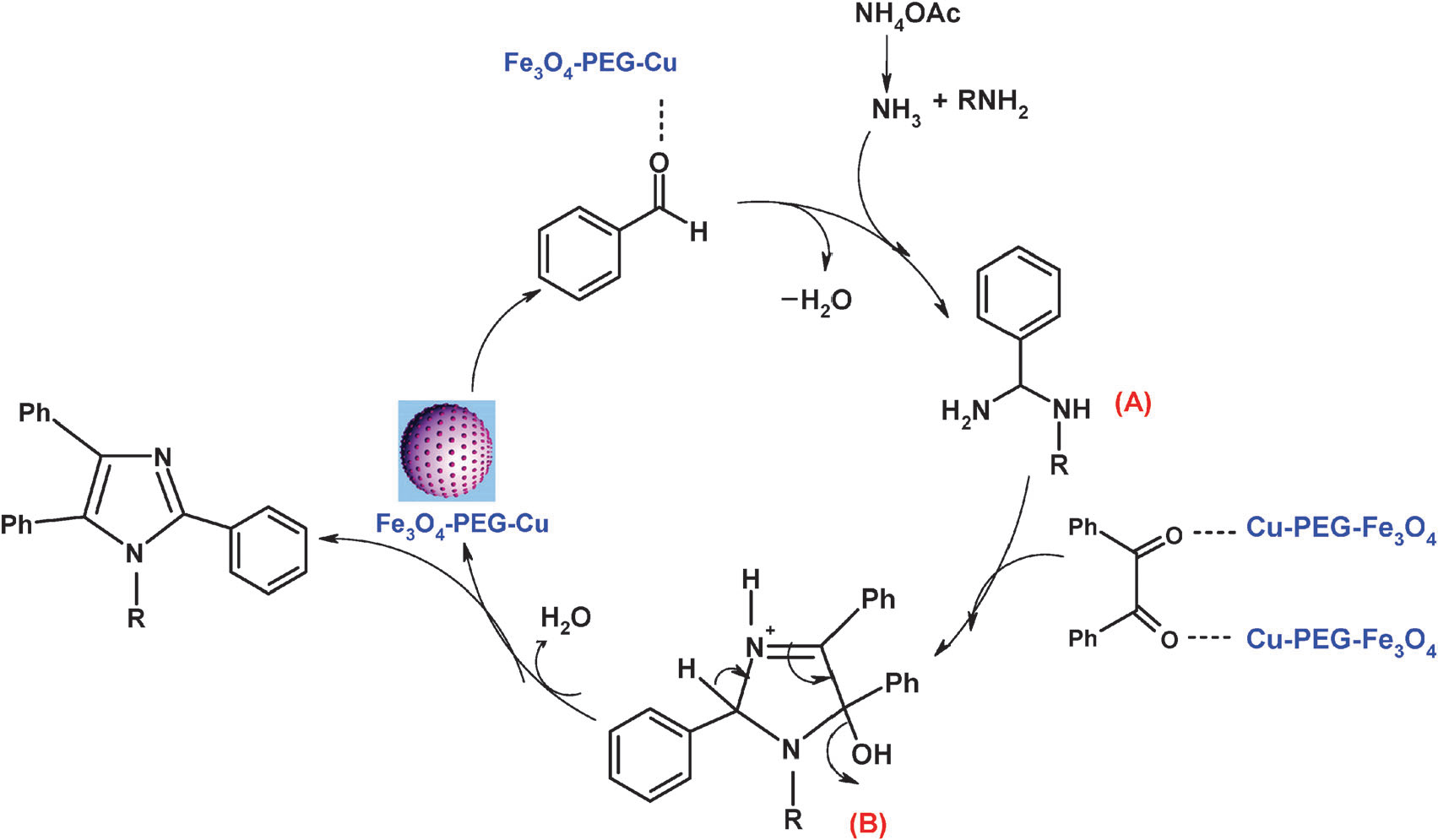
Proposed mechanism for the formation of 1,2,4,5-tetrasubstituted/2,4,5-trisubstituted imidazoles from benzil, aldehydes, NH4OAc, and amine in the presence of Fe3O4–PEG–Cu.
CAS number: 132206-99-6
N-Phenyl-2-cyclopentyl-1H-imidazo(4,5-C)quinolin-4-amine is a chemical compound that belongs to the class of 1H-imidazo[4, 5-c]quinolin-4-amine derivatives. These compounds have garnered attention in medicinal chemistry due to their potential biological activities, particularly as allosteric modulators of the human A3 adenosine receptor (A3AR).
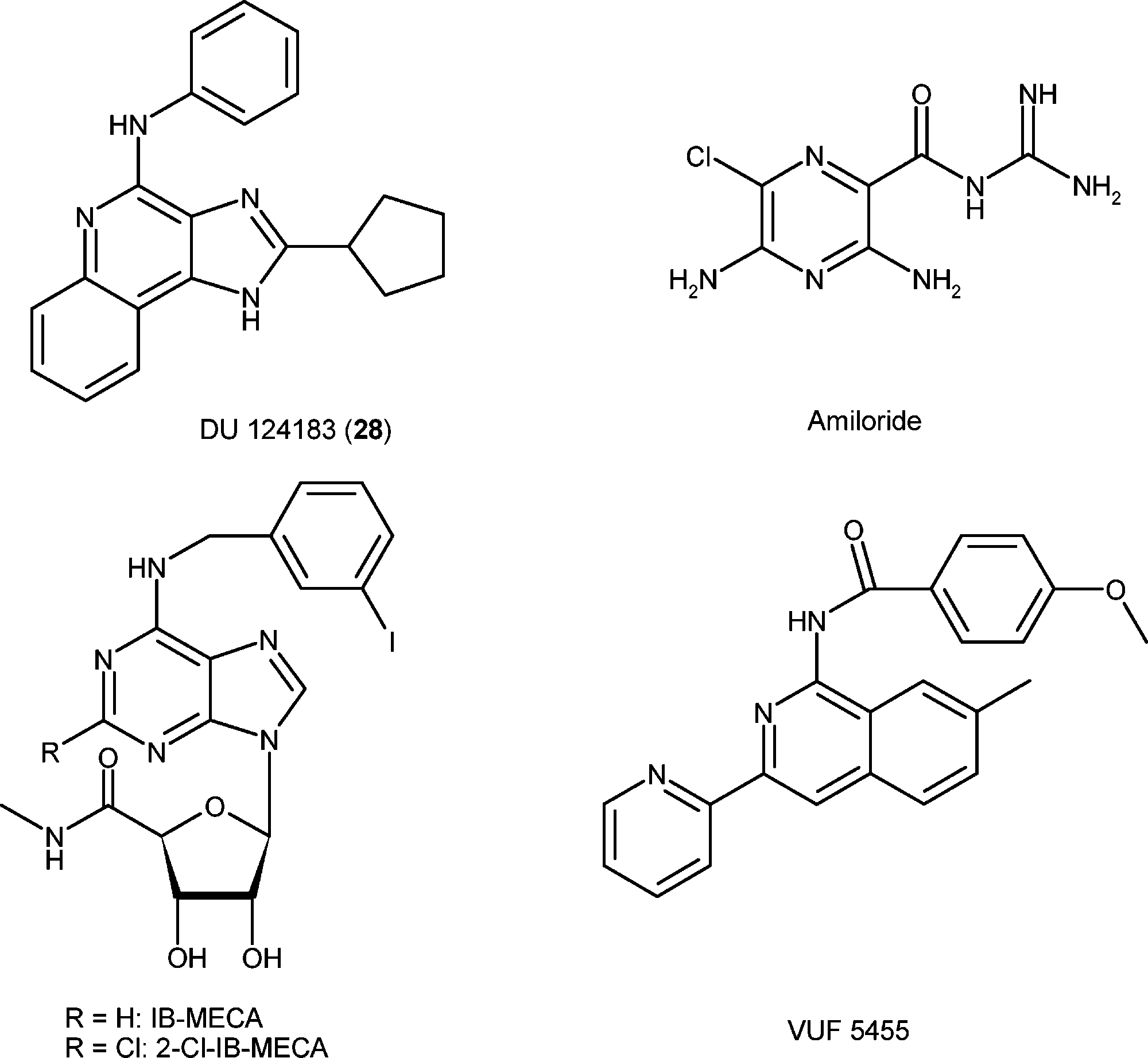
Structures of reference agonists (IB-MECA and 2-Cl-IB-MECA) and allosteric modulators (DU 124183, amiloride, and VUF5455) of the A3AR.
CAS number: 1330-20-7
Xylene, [mixed isomers] appears as a clear colorless liquid with a characteristic aromatic odor consisting of a mixture of the three isomers (ortho-, meta- and para-).
![UV-vis spectra (a) and emission spectra (b) of ASDSN chromophore at concentration of 10▔5 M in different solvents. The photographs of the chromophore in different solutions [from left to right, xylene, dichloromethane (DCM), ethyl acetate (ETA), ethanol, and dimethyl formamide (DMF)] were taken under natural daylight simulator (D65) lamps (top image), and irradiation of A-Class UV lamps (bottom image) (c).](http://www.wlxkc.cn/picture/3214957_07.png)
UV-vis spectra (a) and emission spectra (b) of ASDSN chromophore at concentration of 10▔5 M in different solvents. The photographs of the chromophore in different solutions [from left to right, xylene, dichloromethane (DCM), ethyl acetate (ETA), ethanol, and dimethyl formamide (DMF)] were taken under natural daylight simulator (D65) lamps (top image), and irradiation of A-Class UV lamps (bottom image) (c).
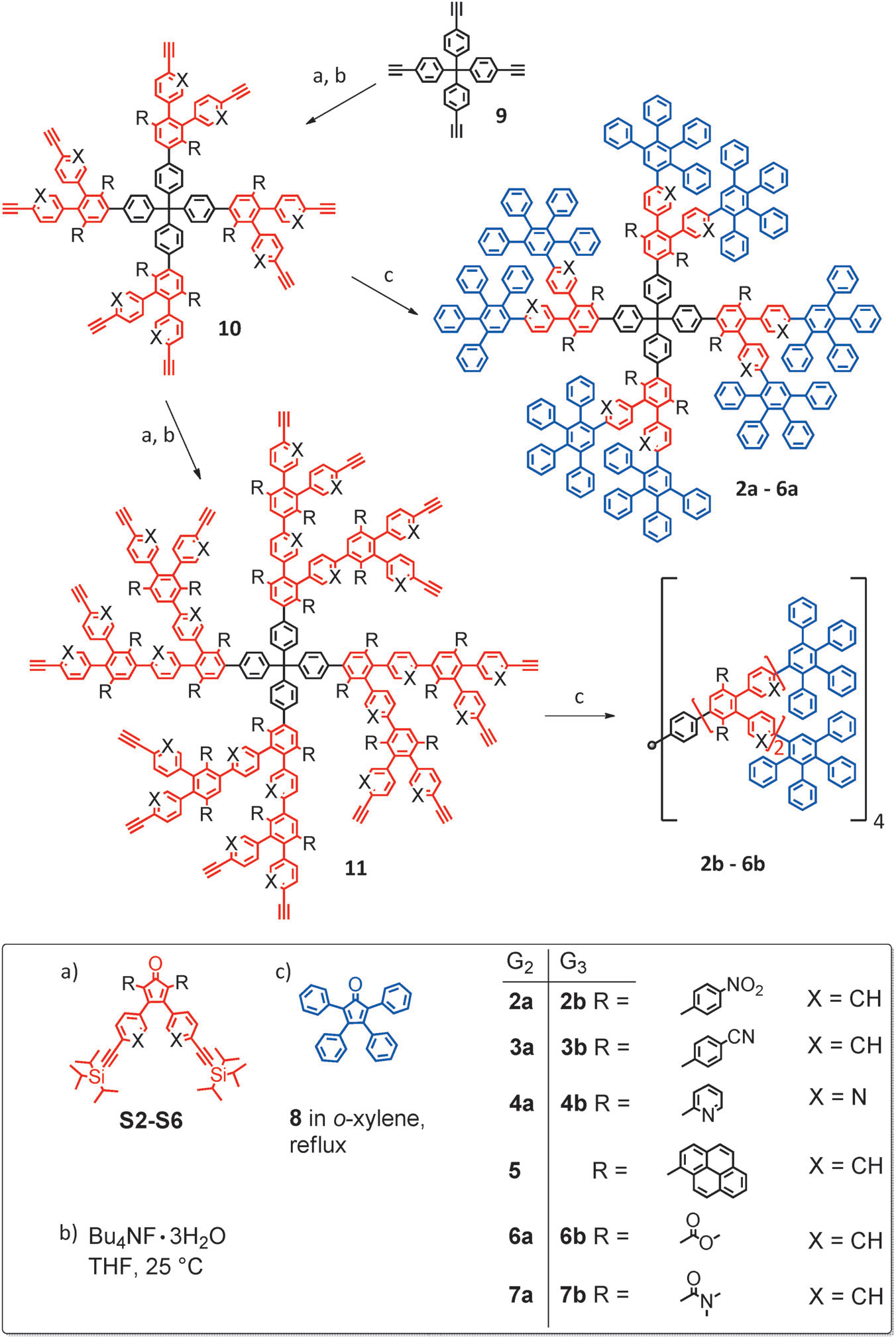
Synthetic pathway towards substituted polyphenylene dendrimers.
CAS number: 13311-84-7
Flutamide is a monocarboxylic acid amide and a member of (trifluoromethyl)benzenes. It has a role as an androgen antagonist and an antineoplastic agent.
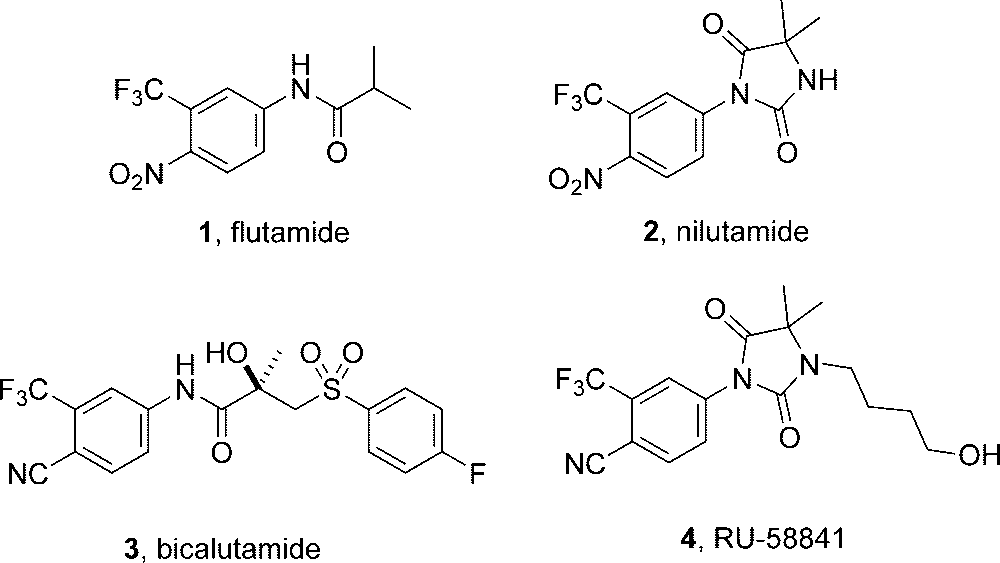
Known nonsteroidal androgen receptor antagonists.