Chemicals list & Research Gallery
CAS number: 104987-11-3
Tacrolimus (also FK-506 or Fujimycin) is an immunosuppressive drug whose main use is after organ transplant to reduce the activity of the patient's immune system and so the risk of organ rejection. It is also used in a topical preparation in the treatment of severe atopic dermatitis, severe refractory uveitis after bone marrow transplants, and the skin condition vitiligo.
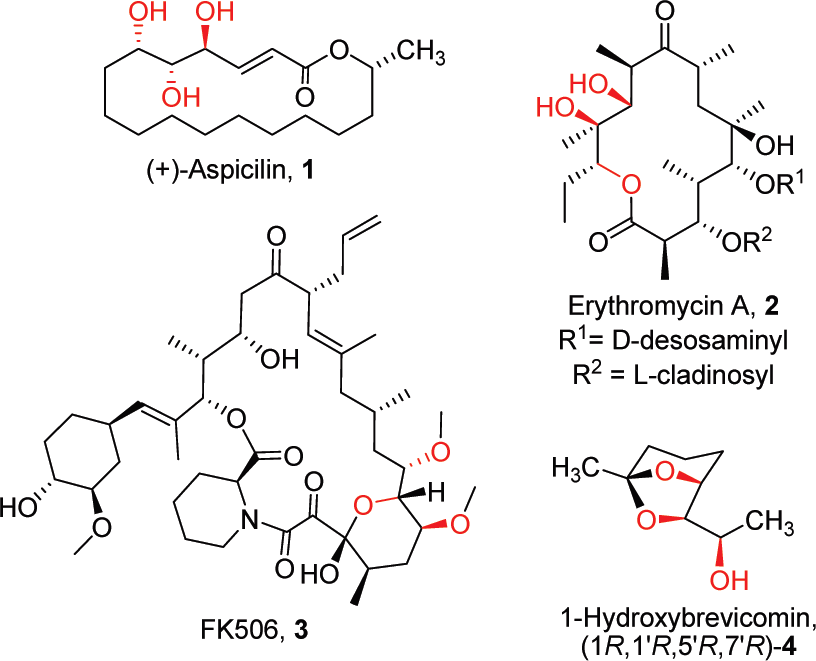
Natural products include or are derived from D- or L-arabinotriol.
CAS number: 105-53-3
Diethyl malonate is a metabolite found in or produced by Saccharomyces cerevisiae.
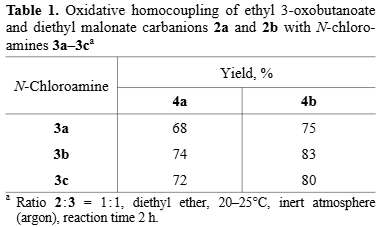
Oxidative homocoupling of ethyl 3-oxobutanoate and diethyl malonate carbanions 2a and 2b with N-chloro-amines 3a–3
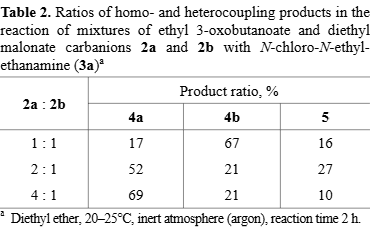
Ratios of homo- and heterocoupling products in the reaction of mixtures of ethyl 3-oxobutanoate and diethyl malonate carbanions 2a and 2b with N-chloro-N-ethyl-ethanamine (3a)
CAS number: 105-56-6
Ethyl cyanoacetate appears as a colorless liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
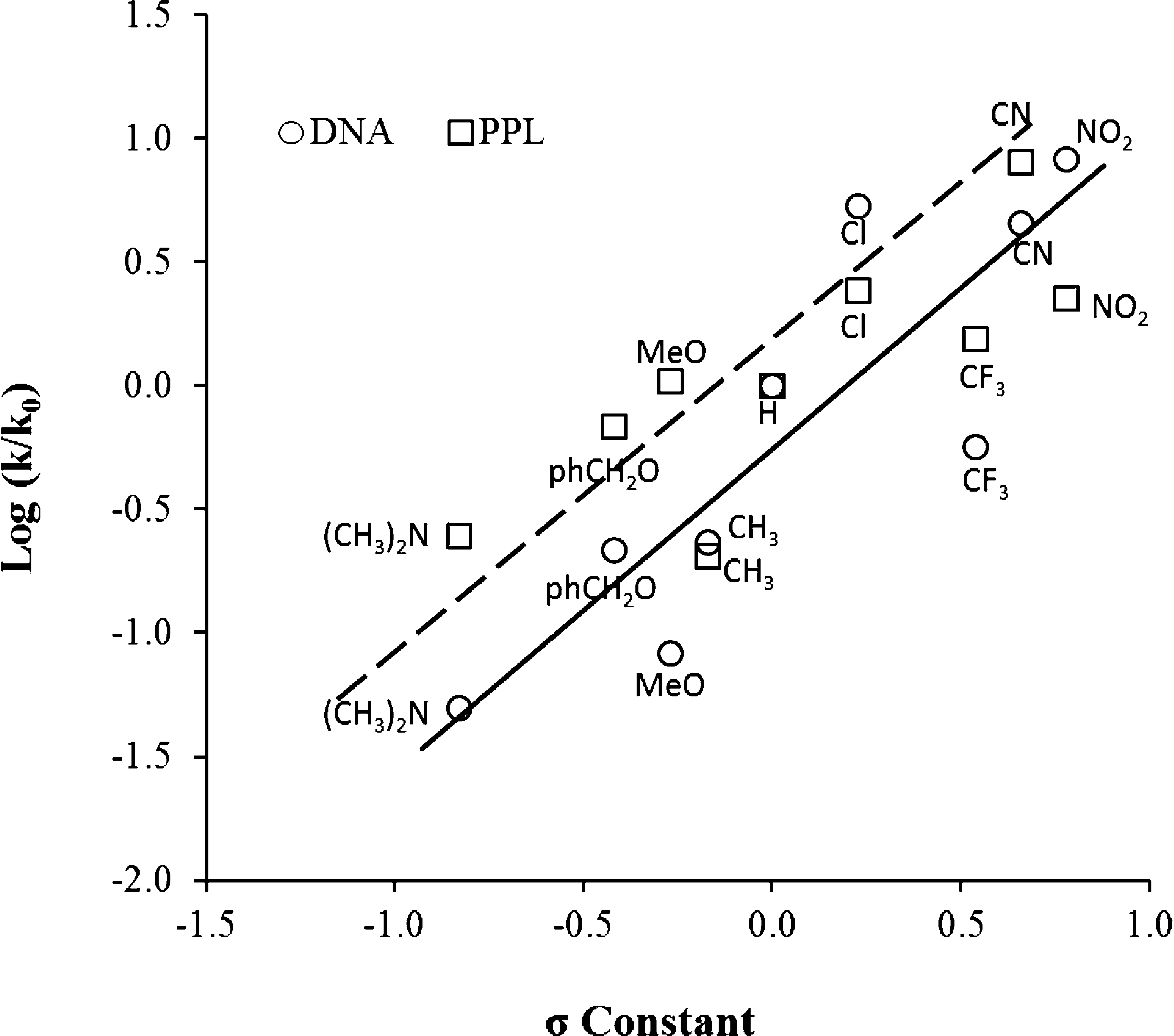
Hammett plots of the Knoevenagel condensation reaction of para-substituted benzaldehydes with ethyl cyanoacetate catalyzed by sodium salt of DNA fragments (circles) and PPL (squares).
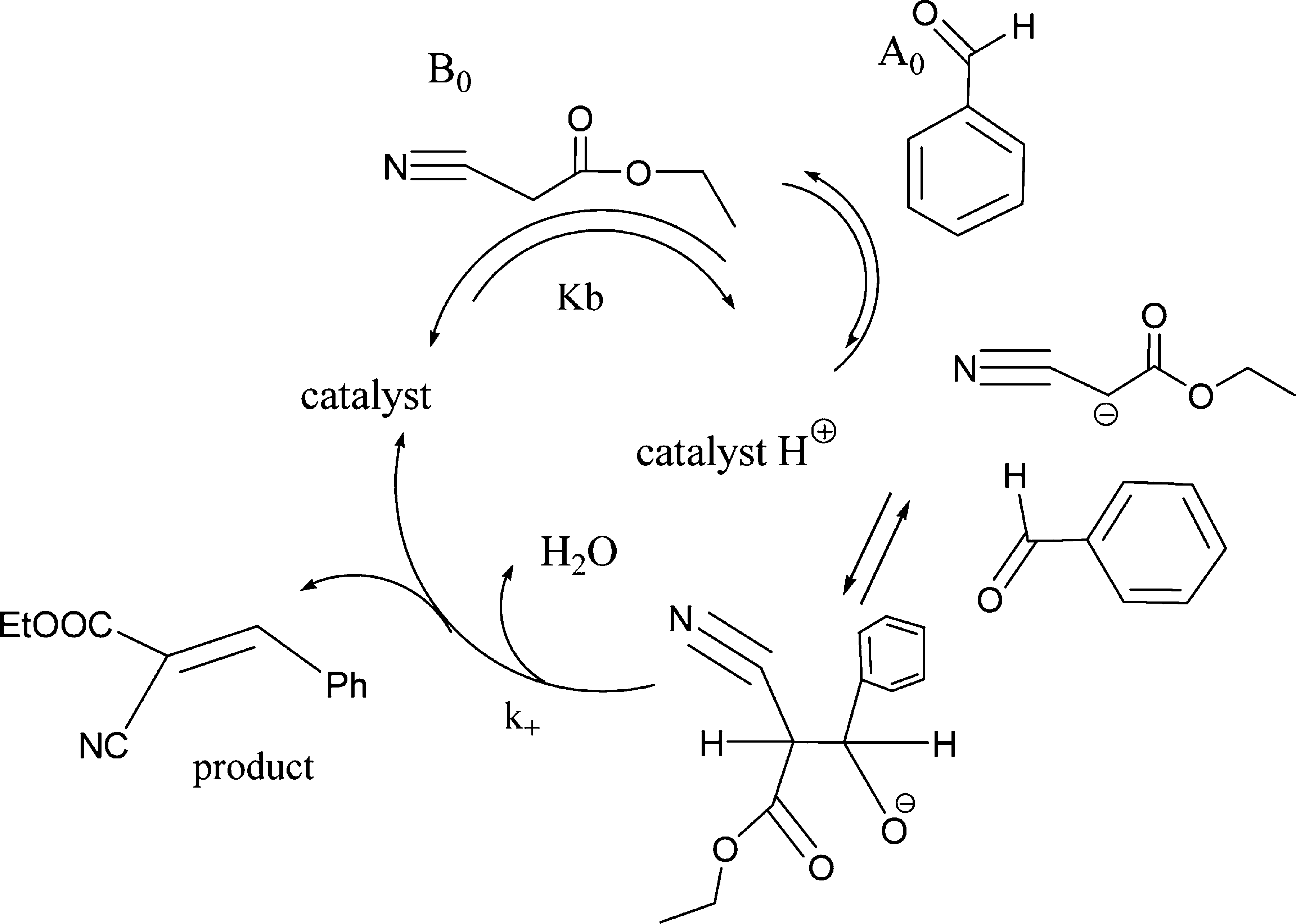
Proposed Kinetic Mechanism DNA/RNA-Salts Appears To Progress via the Shown Catalytic Cycle
CAS number: 10534-59-5
Tetrabutylammonium acetate is an organic salt used as a catalyst, curing agent, and initiator in polymer synthesis, and as a phase-transfer catalyst. It is also utilized in the preparation of ionic liquids and in analytical chemistry. It is a white to off-white solid, highly hygroscopic, and can be used in organic synthesis, electrochemistry, and biotechnology applications.
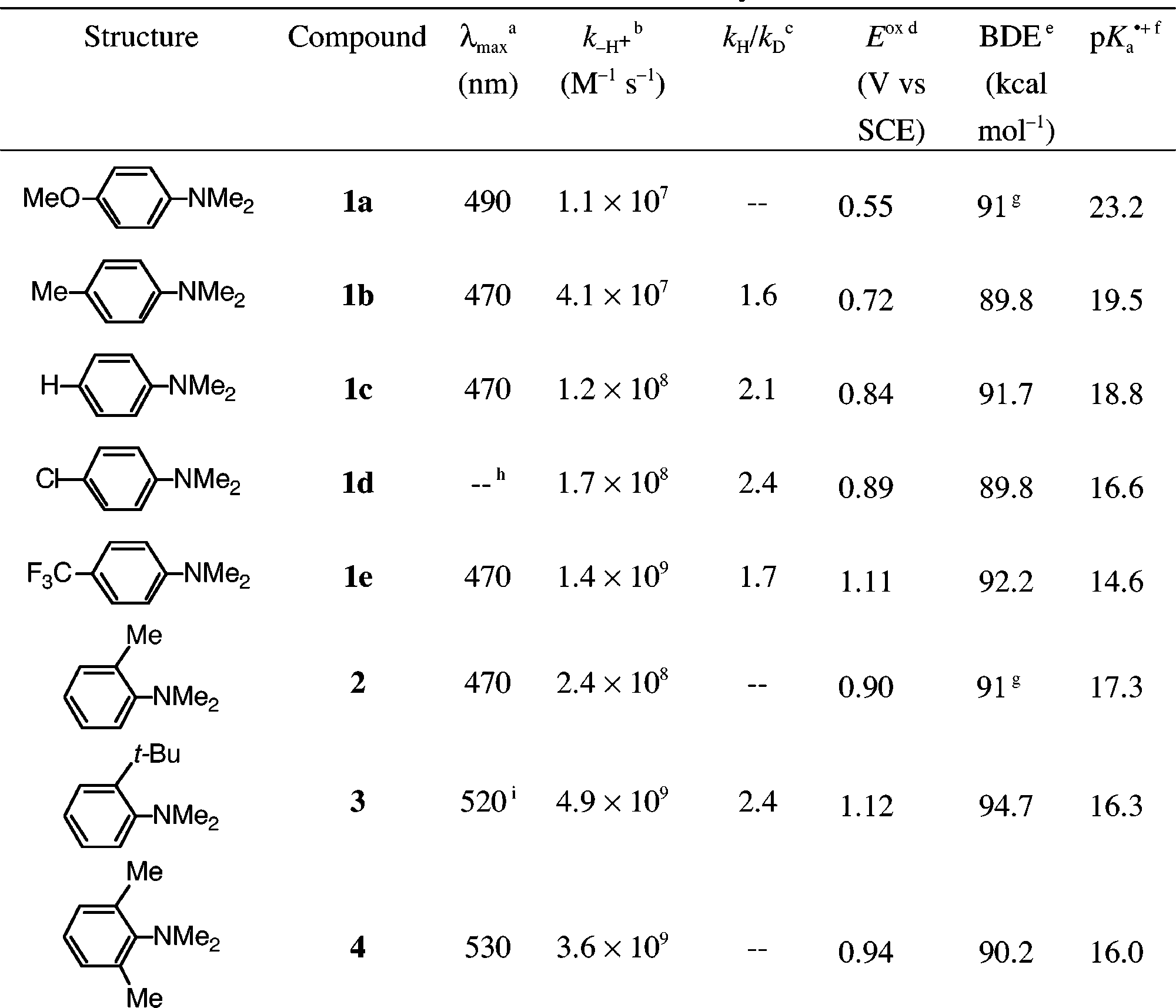
Spectral, Kinetic, and Thermodynamic Properties Related to Deprotonation of Aromatic Amine Radical Cations with Tetrabutylammonium Acetate as the Base in Acetonitrile Containing 0.5 m Water and 0.5 m Tetrabutylammonium Perchlorate
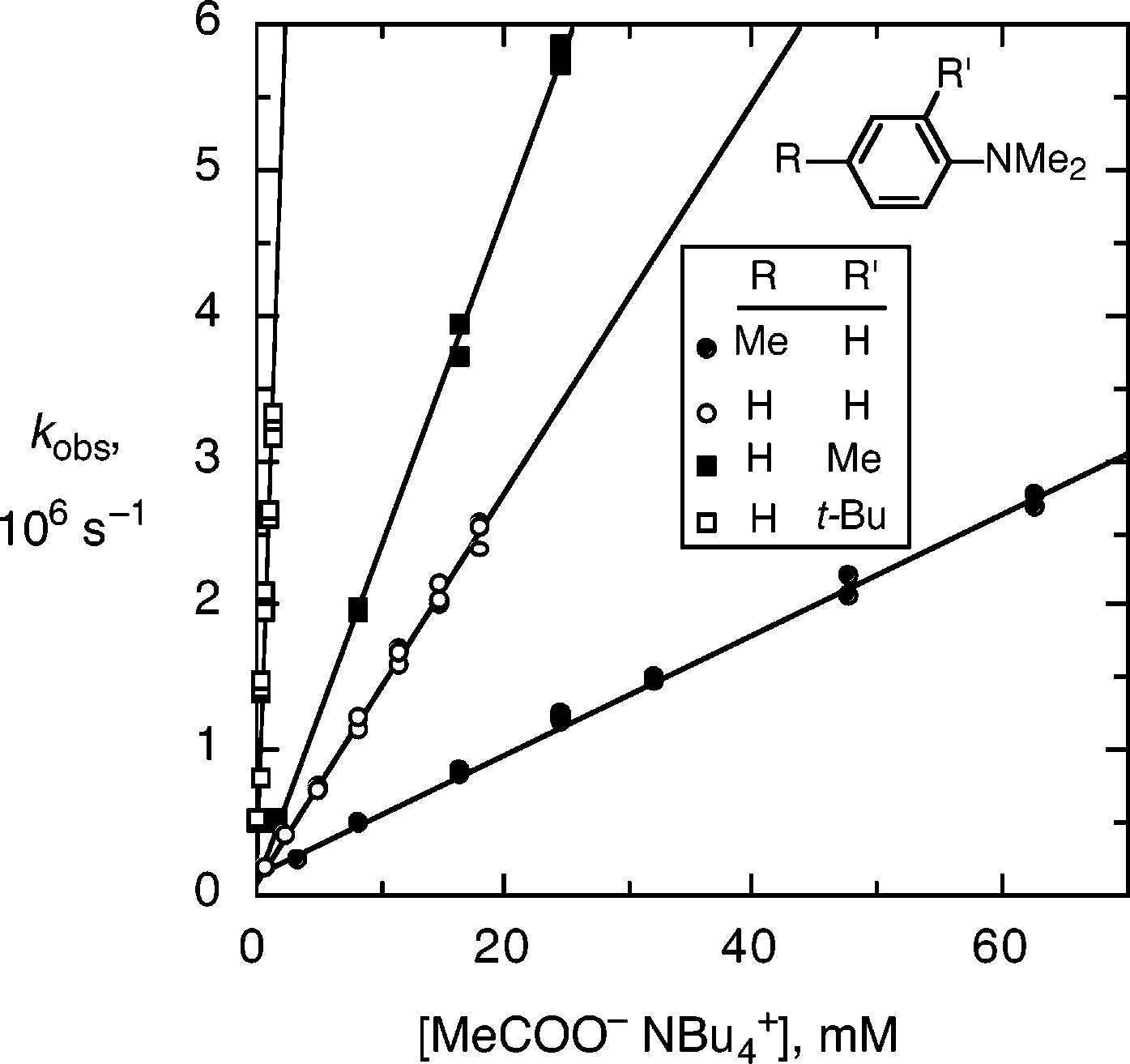
Plots of observed rate of pseudo-first-order decay, kobs, versus concentration of tetrabutylammonium acetate for deprotonation of the radical cations of (open circles) N,N-dimethylaniline, 1c, and N,N,-dimethylaniline with (filled circles) a 4-methyl substituent, 1b, (filled squares) a 2-methyl substituent, 2, and (open squares) a 2-tert-butyl substituent, 3, in acetonitrile with 0.5 m water and 0.5 m tetramethylammonium perchlorate, at room temperature.
CAS number: 10549-76-5
Tetrabutylammonium is a chemical compound characterized by a positively charged nitrogen atom bonded to four butyl groups (C4H9), forming a quaternary ammonium cation. It's often used in the form of salts with various anions, such as bromide, chloride, fluoride, or perchlorate.
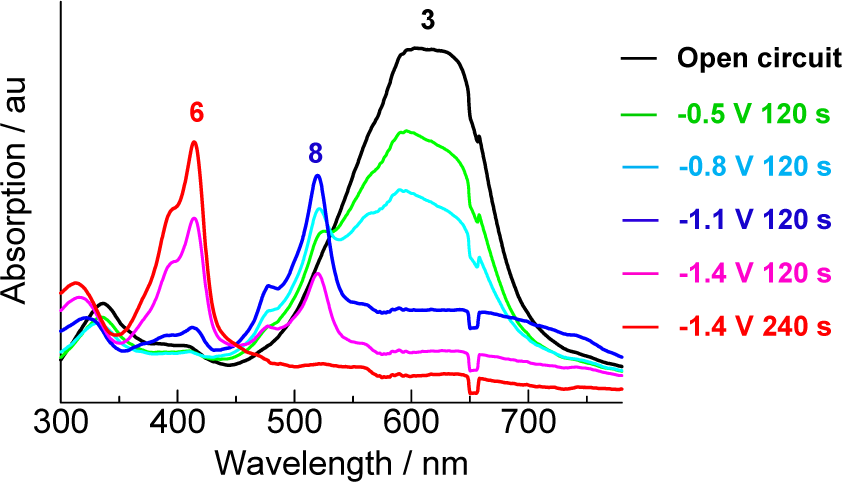
Electrochromic behavior of 3 in THF with tetrabutylammonium perchlorate as the supporting electrolyte at 0.0, −0.5, −0.8, −1.1, −1.4 (V vs Ag/Ag+ as a reference electrode). Platinum was used as the working and counter electrodes.
CAS number: 1056634-68-4
Momelotinib is a small molecule Janus kinase inhibitor that is used in the treatment of intermediate or high risk, primary or secondary myelofibrosis. Momelotinib is associated with transient and usually mild elevations in serum aminotransferase levels during therapy and has also been linked to instances of clinically apparent acute liver injury including reactivation of hepatitis B.
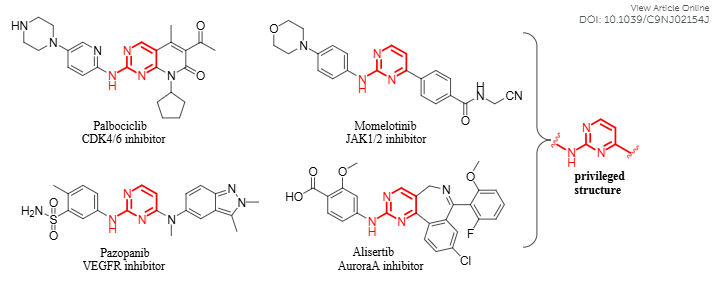
Structures of representative kinase inhibitors containing aminopyrimidine nuclei: Palbociclib, Momelotinib, Pazopanib, Alisertib
CAS number: 1056970-88-7
Spiro[2.4]heptane-4,7-dione is a chemical compound features a spirocyclic structure, where a cyclopropane ring is fused to a cycloheptane ring at a single carbon atom. Specifically, the 4 and 7 positions of the cycloheptane ring are ketones (dione).
![Preparation of Spiro[2.4]heptane-4,7-dione Derivatives and Light-Driven Cyclopropanation](http://www.wlxkc.cn/picture/1227973_04.png)
Preparation of Spiro[2.4]heptane-4,7-dione Derivatives and Light-Driven Cyclopropanation
CAS number: 105798-74-1
Clitocine is a N-glycosyl compound.
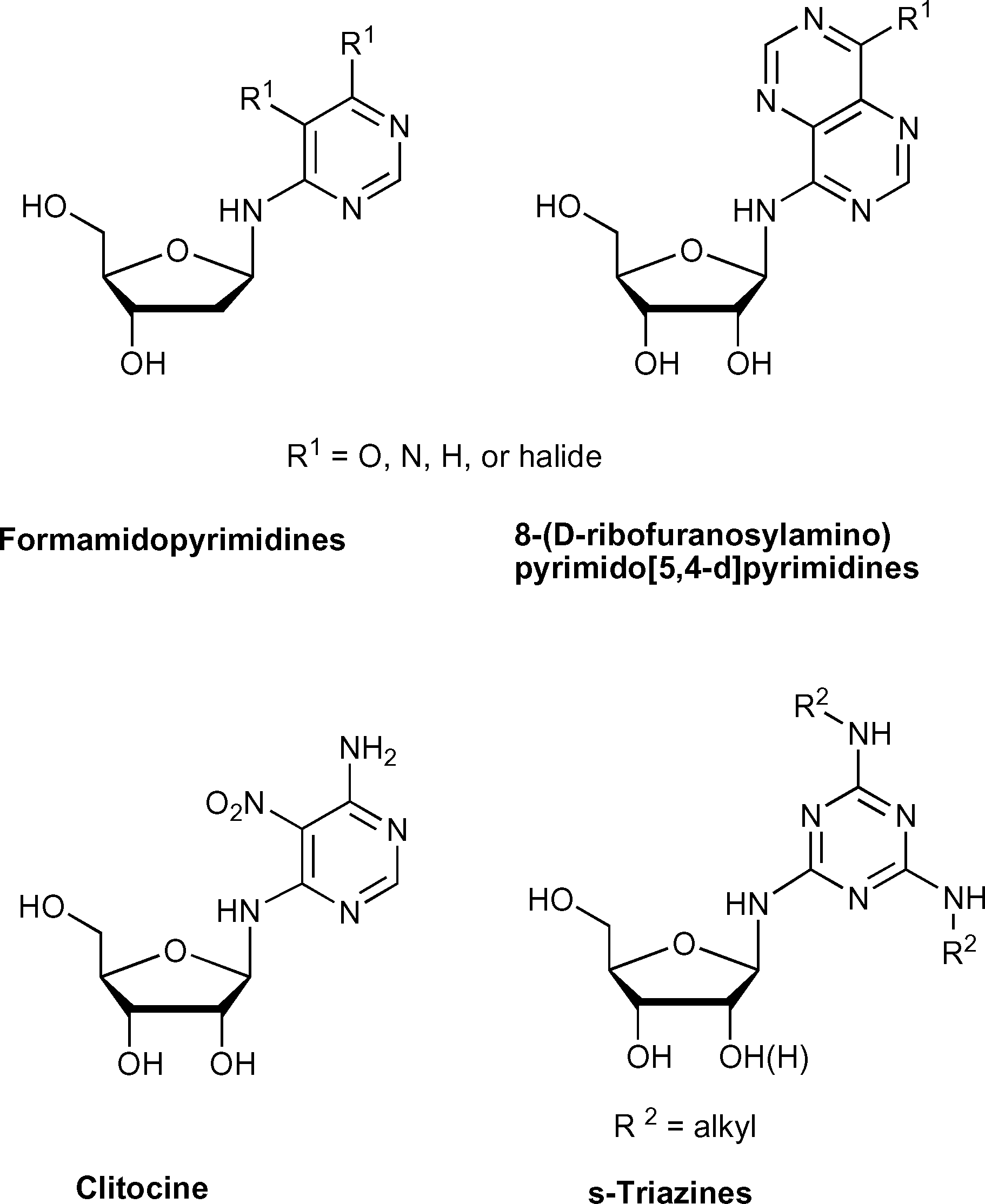
Exocyclic amino nucleosides: Clitocine, s-triazines.
CAS number: 106-51-4
1,4-benzoquinone is the simplest member of the class of 1,4-benzoquinones, obtained by the formal oxidation of hydroquinone to the corresponding diketone. It is a metabolite of benzene. It has a role as a cofactor, a human xenobiotic metabolite and a mouse metabolite.
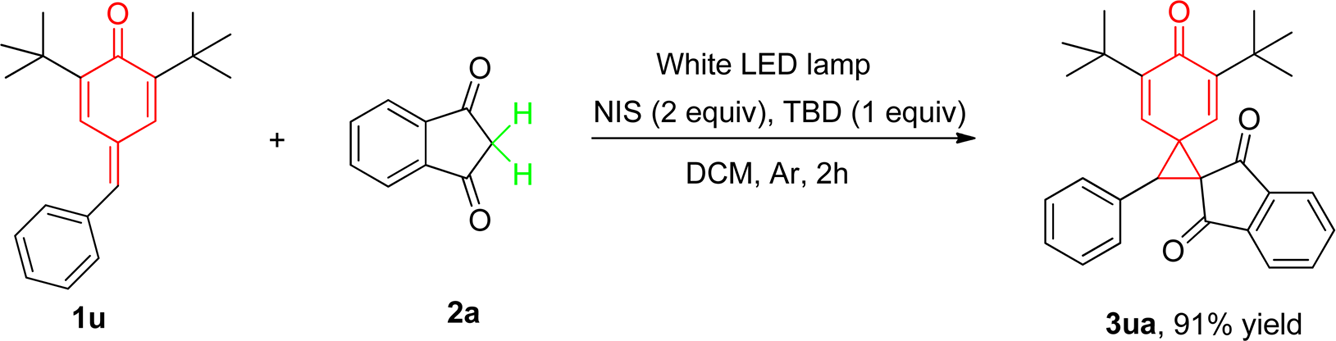
Spirocyclopropanation Reaction of p-Quinone Methide
CAS number: 106-96-7
3-bromopropyne appears as a colorless to light yellow liquid substance with a sharp odor. Flash point 65 °F. Denser than water and insoluble in water. Vapors are heavier than air. May be irritating to skin and eyes. Used to make other chemicals. It may decompose explosively with mild shock.
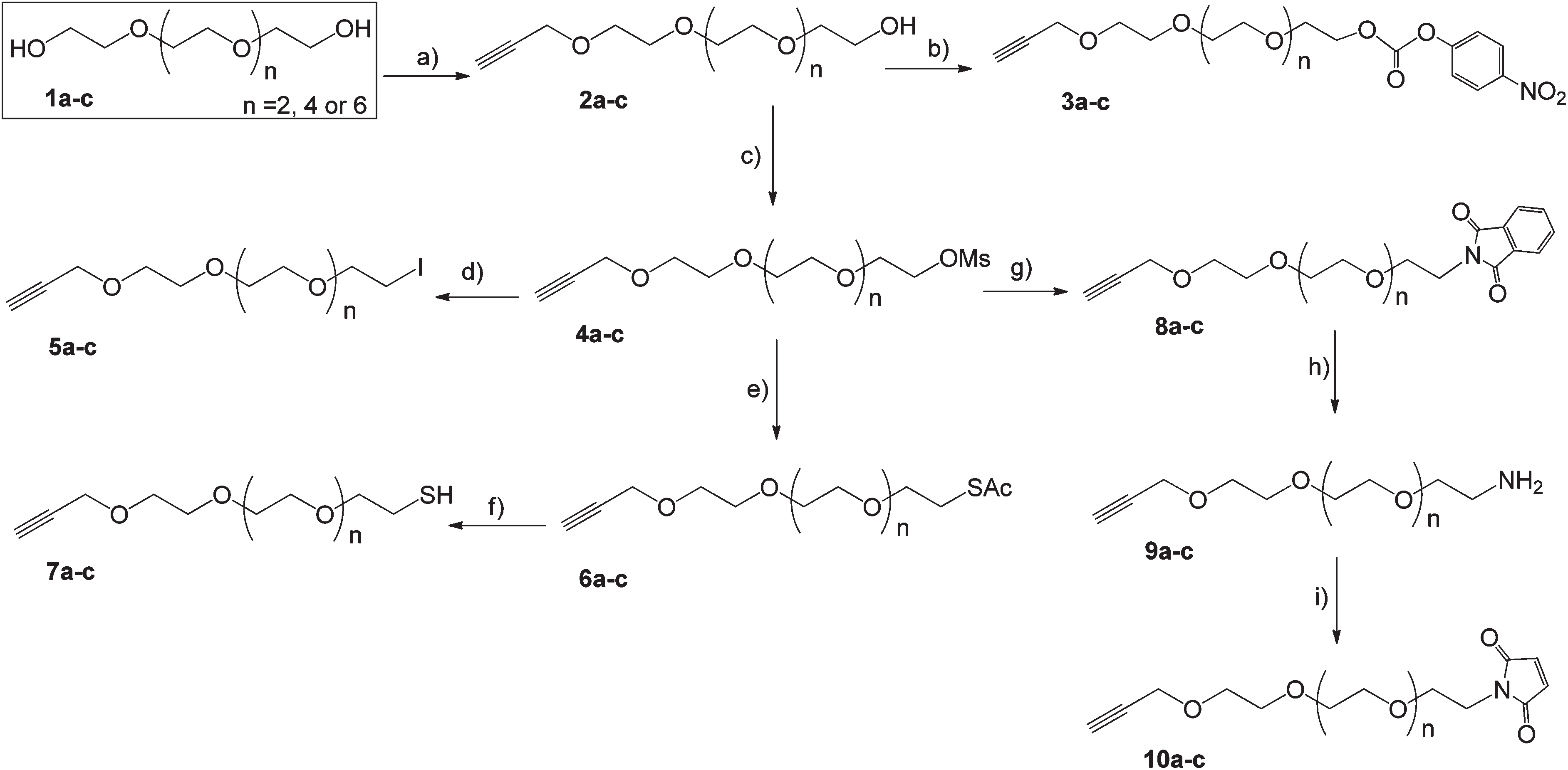
Synthesis of alkyne terminated heterobifunctionalized OEG linkers. Reagents and conditions: (a) Propargyl bromide, NaH, THF, 0 °C–RT–60 °C, 15 h; (b) 4-nitrophenyl chloroformate, pyridine, MeCN, RT, 15 h; (c) MsCl, Et3N, DCM, 0 °C–RT, 3.5 h; (d) NaI, acetone, 65 °C, 15 h; (e) KSAc, DMF, RT, 15 h; (f) LAH, THF, −10 °C–RT, 3 h; (g) Potassium phthalimide, DMF, 110 °C, 15 h; (h) Hydrazine, EtOH, 60 °C, 3 h; (i) N-methoxycarbonyl maleimide, Sat. aq NaHCO3, 0 °C–RT, 2 h.