Chemicals list & Research Gallery
CAS number: 188-52-3
Dibenzo[c,g]phenanthrene is a polycyclic aromatic hydrocarbon (PAH) composed of four fused benzene rings. It is also known as pentahelicene.
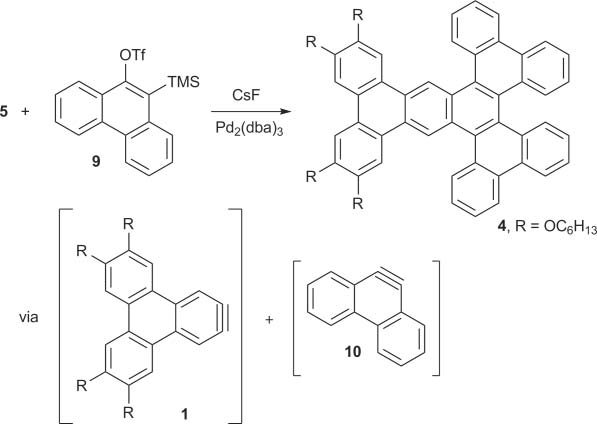
Synthesis of substituted pentahelicene 4.
CAS number: 1885-38-7
(2E)-3-Phenyl-2-propenenitrile, also known as cinnamonitrile or (E)-cinnamyl nitrile. Structurally, it consists of a phenyl group (C₆H₅–) attached to a propenenitrile chain (CH=CH–CN), where the double bond between the second and third carbon atoms is in the E (trans) configuration. This gives the molecule a linear geometry across the double bond, contributing to its stability and unique chemical reactivity. It appears as a colorless to pale yellow liquid and is used primarily as an intermediate in organic synthesis, including the production of pharmaceuticals, agrochemicals, and fragrances. Its nitrile group contributes to moderate polarity and can participate in various chemical transformations such as reduction, hydrolysis, or cyclization.
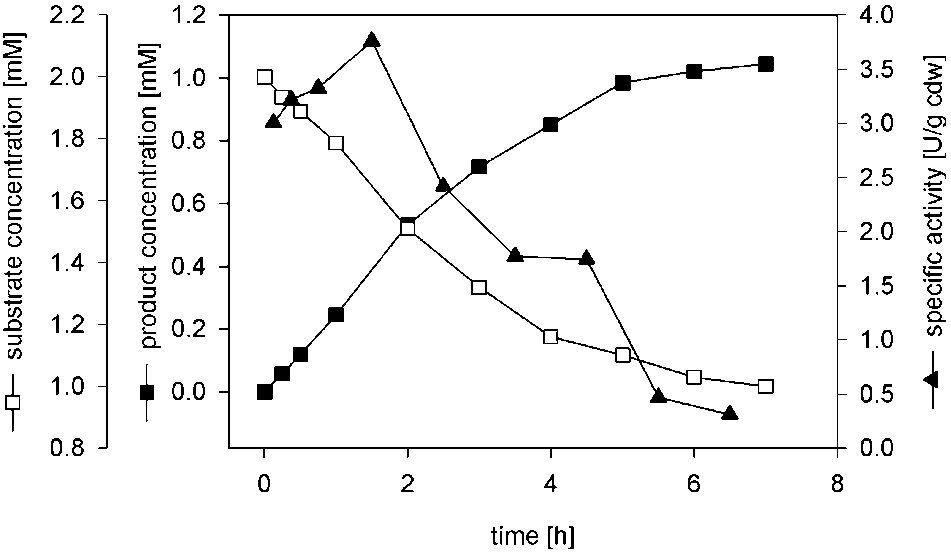
Biotransformation of cinnamonitrile using E. coli JM101 (pTEZ30) in shake flasks. Reaction was performed usingrestingcells (1.29 gcdw/L) in 20-mL shake flasks.
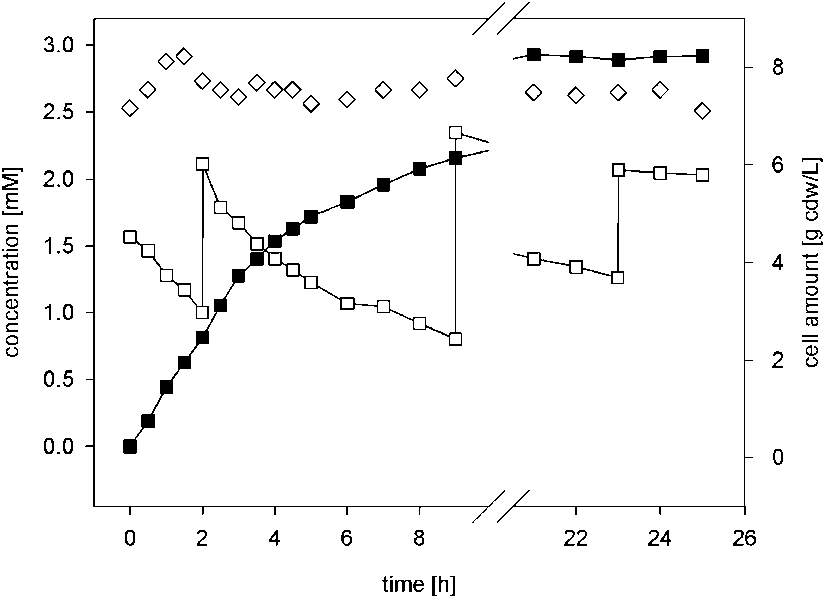
Biotransformation of cinnamonitrile using E. coli JM101 (pTEZ30) in a bioreactor on a 2-L scale.
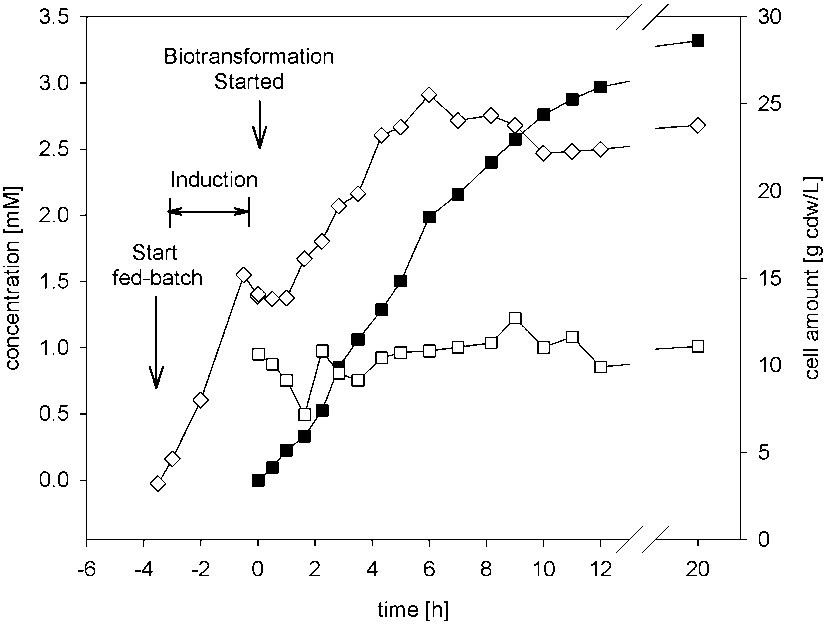
Biotransformation of cinnamonitrile using E. coli JM101 (pTEZ30) in a bioreactor with a 30-L workingvol-ume.
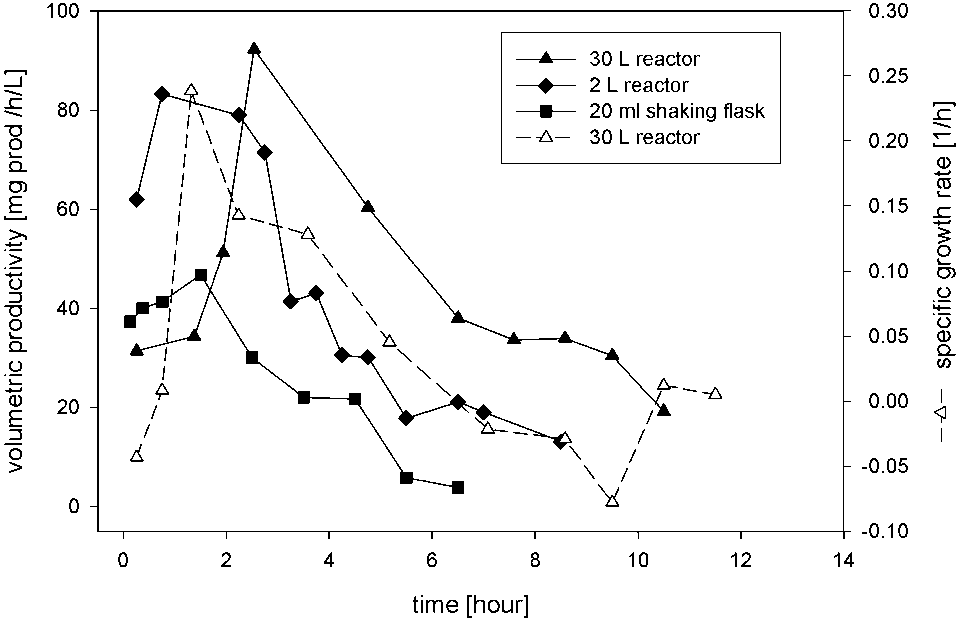
Volumetric productivities of biotransformation of cinnamonitrile using E. coli JM101 (pTEZ30) in shake-flasks, and in reactors on 2-L and 30-L scales, and the specific growth rate of the cells in a 30-L reactor.
CAS number: 18908-66-2
2-Ethylhexyl bromide, also known as 1-bromo-2-ethylhexane, is a colorless to pale yellow liquid. It is an organobromine compound derived from 2-ethylhexanol and is primarily used as an intermediate in organic synthesis, particularly in the production of pharmaceuticals, agrochemicals, and specialty chemicals.
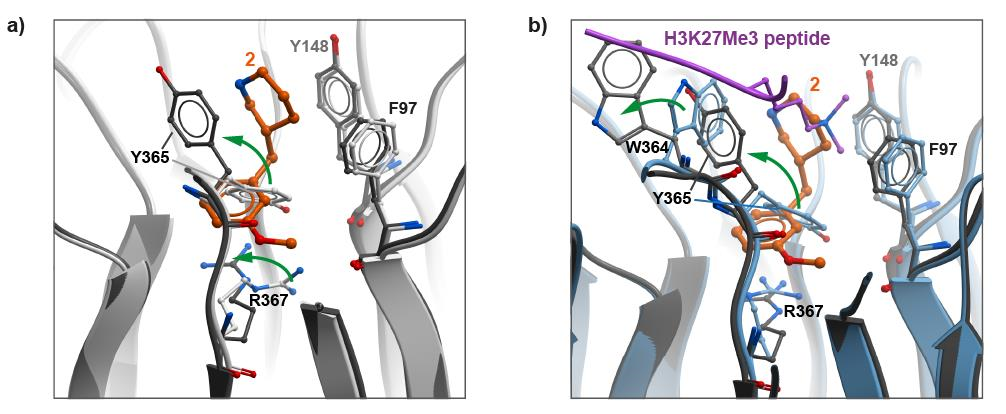
Conformational changes observed upon binding of compound 2.
CAS number: 1892-57-5
1-Ethyl-3-(3′-dimethylaminopropyl)carbodiimide, commonly abbreviated as EDC or EDCI, is a water-soluble carbodiimide reagent widely used in organic and bioconjugation chemistry, especially for peptide synthesis and crosslinking reactions.
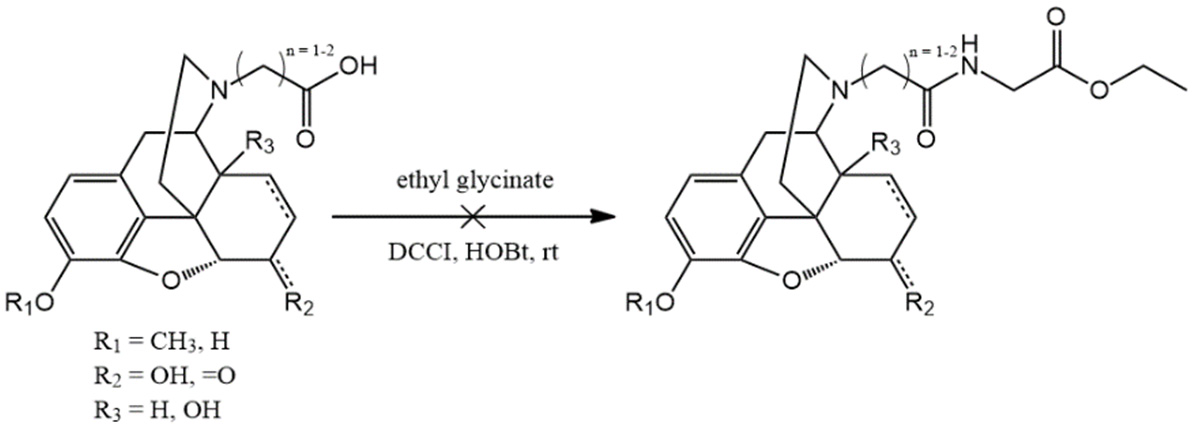
Attempted amino acid coupling: glycine ethyl ester, N,N'-dicyclohexyl carbodiimide (DCCI) or 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDAC), 1-hydroxybenzotriazole (HOBt), water, room temperature.
CAS number: 189508-31-4
4-[(Z,13R,14R)-13,14-dihydroxytriacont-17-enyl]-2-methyl-2H-furan-5-one is a long-chain fatty acid–derived lactone featuring a 30-carbon aliphatic backbone with a cis double bond at the 17th carbon and two stereospecific hydroxyl groups at C13 and C14 in the R configuration. The terminal portion of this chain is linked to the 4-position of a 2-methylfuran-5-one ring, a five-membered lactone with a methyl substituent at C2.
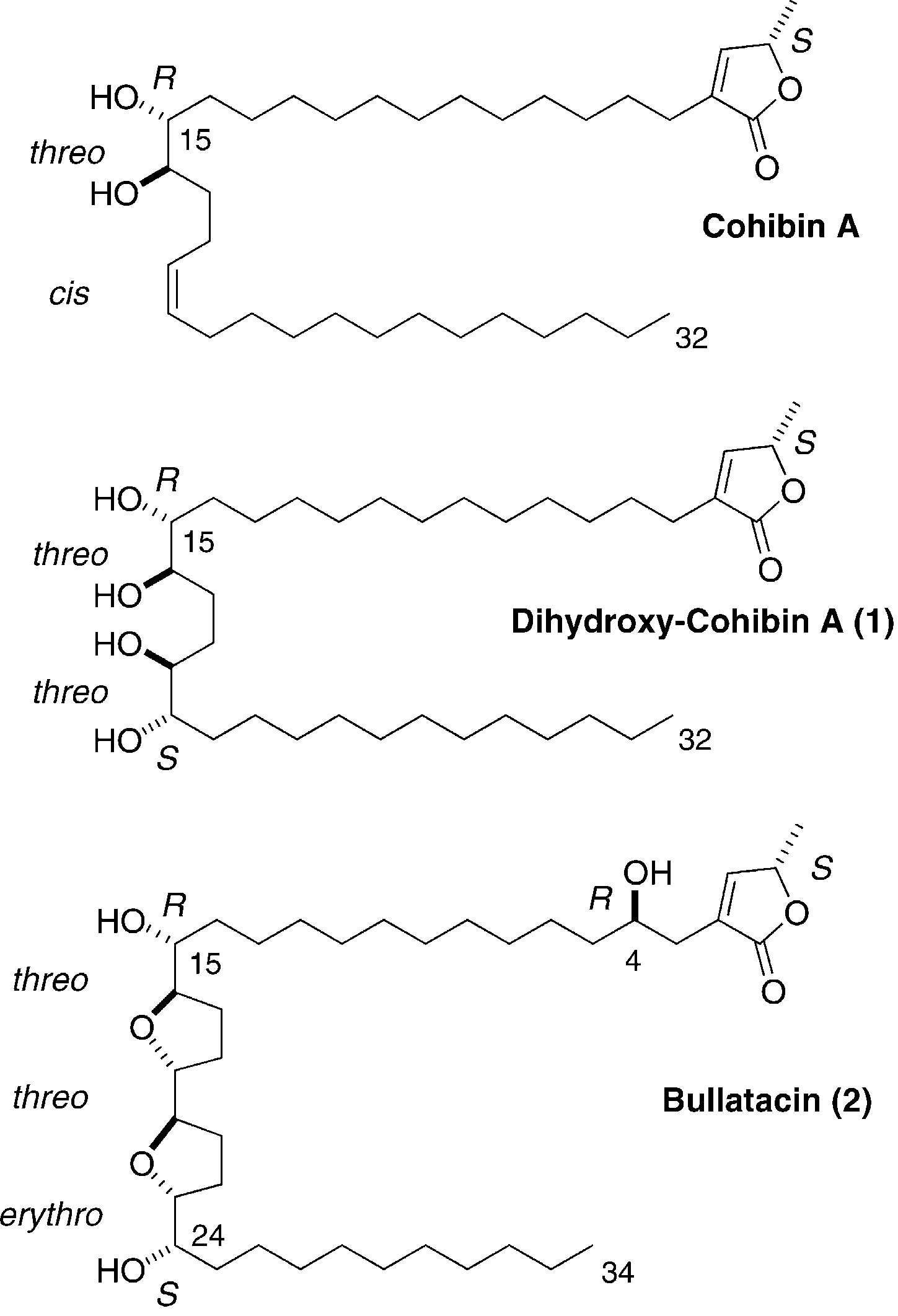
Structure of cohibin A, synthetic dihydroxy-cohibin A (1) and bullatacin (2).
CAS number: 190781-74-9
Amphidinol 3 (AM3) is a naturally occurring compound, specifically a polyene macrolide, produced by the marine dinoflagellate Amphidinium klebsii. It's known for its potent antifungal and hemolytic (blood cell-damaging) activities. AM3 achieves these effects by interacting with and permeabilizing cell membranes, forming pores in the process.
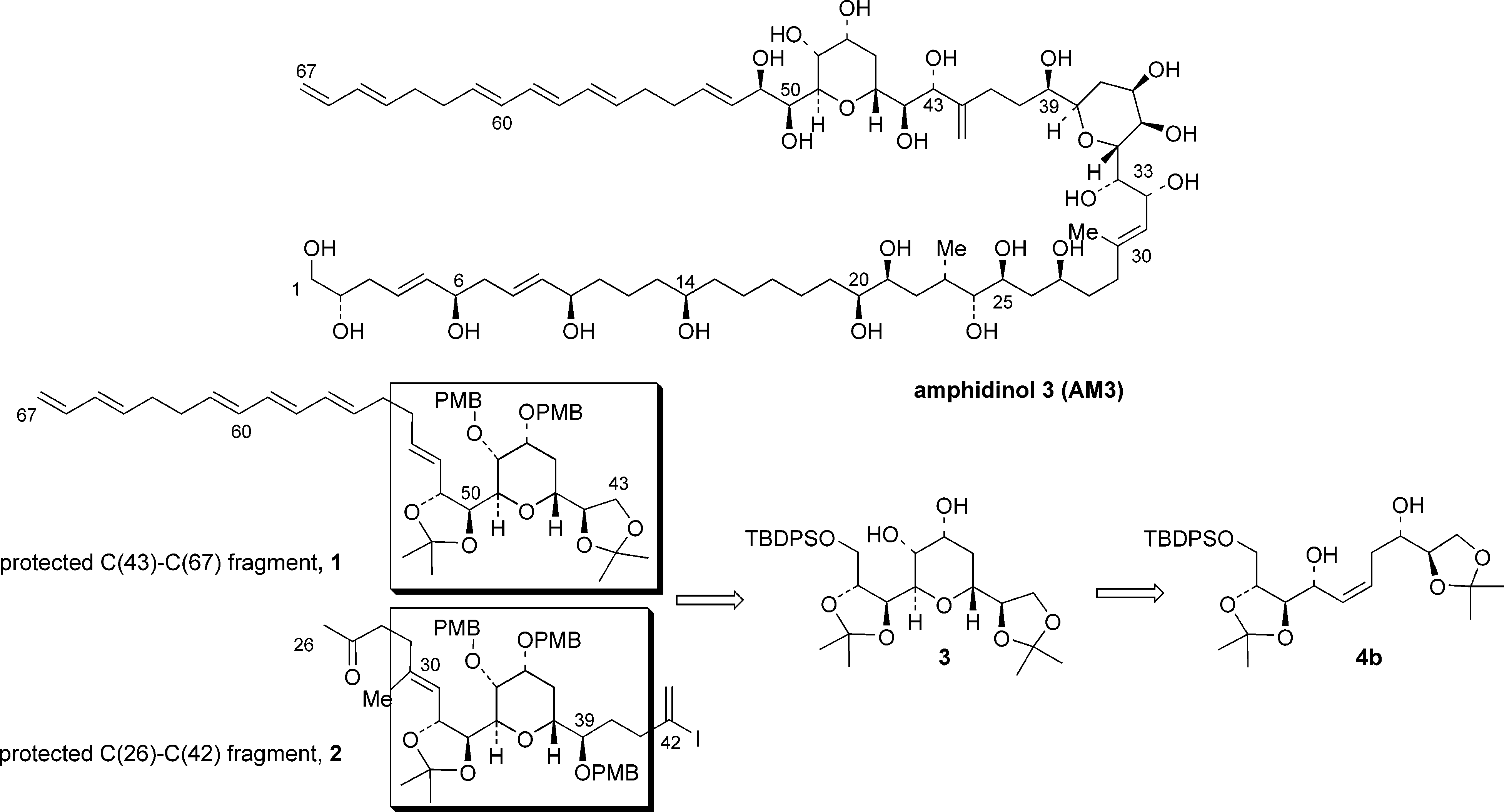
Retrosynthetic analysis (Amphidinol 3).
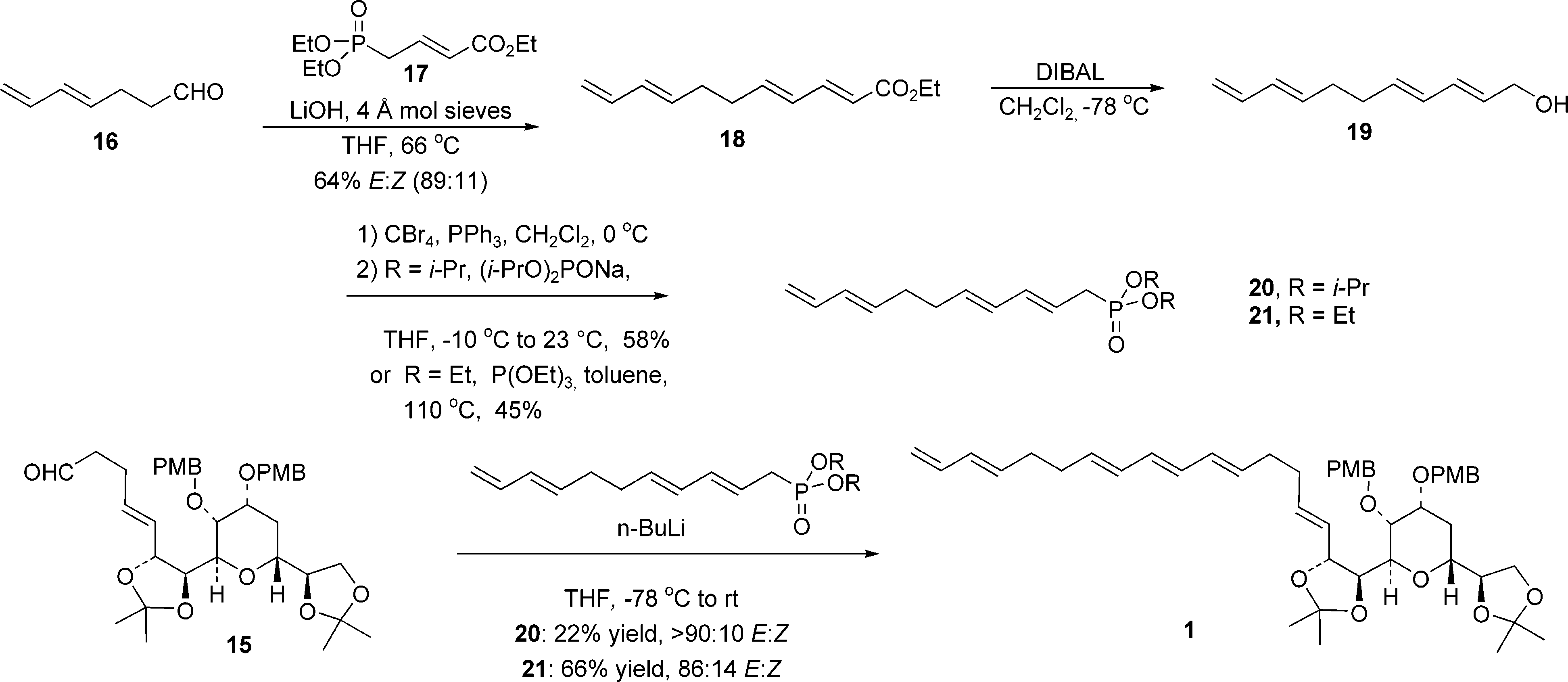
Synthesis of the C(43)-C(67) Fragment of Am3(Amphidinol 3)
CAS number: 191114-48-4
Telithromycin, a semi-synthetic erythromycin derivative, belongs to a new chemical class of antibiotics called ketolides. Ketolides have been recently added to the macrolide-lincosamide-streptogramin class of antibiotics. Similar to the macrolide antibiotics, telithromycin prevents bacterial growth by interfering with bacterial protein synthesis. Telithromycin binds to the 50S subunit of the 70S bacterial ribosome and blocks further peptide elongation. Binding occurs simultaneously at to two domains of 23S RNA of the 50S ribosomal subunit, domain II and V, where older macrolides bind only to one. It is used to treat mild to moderate respiratory infections.
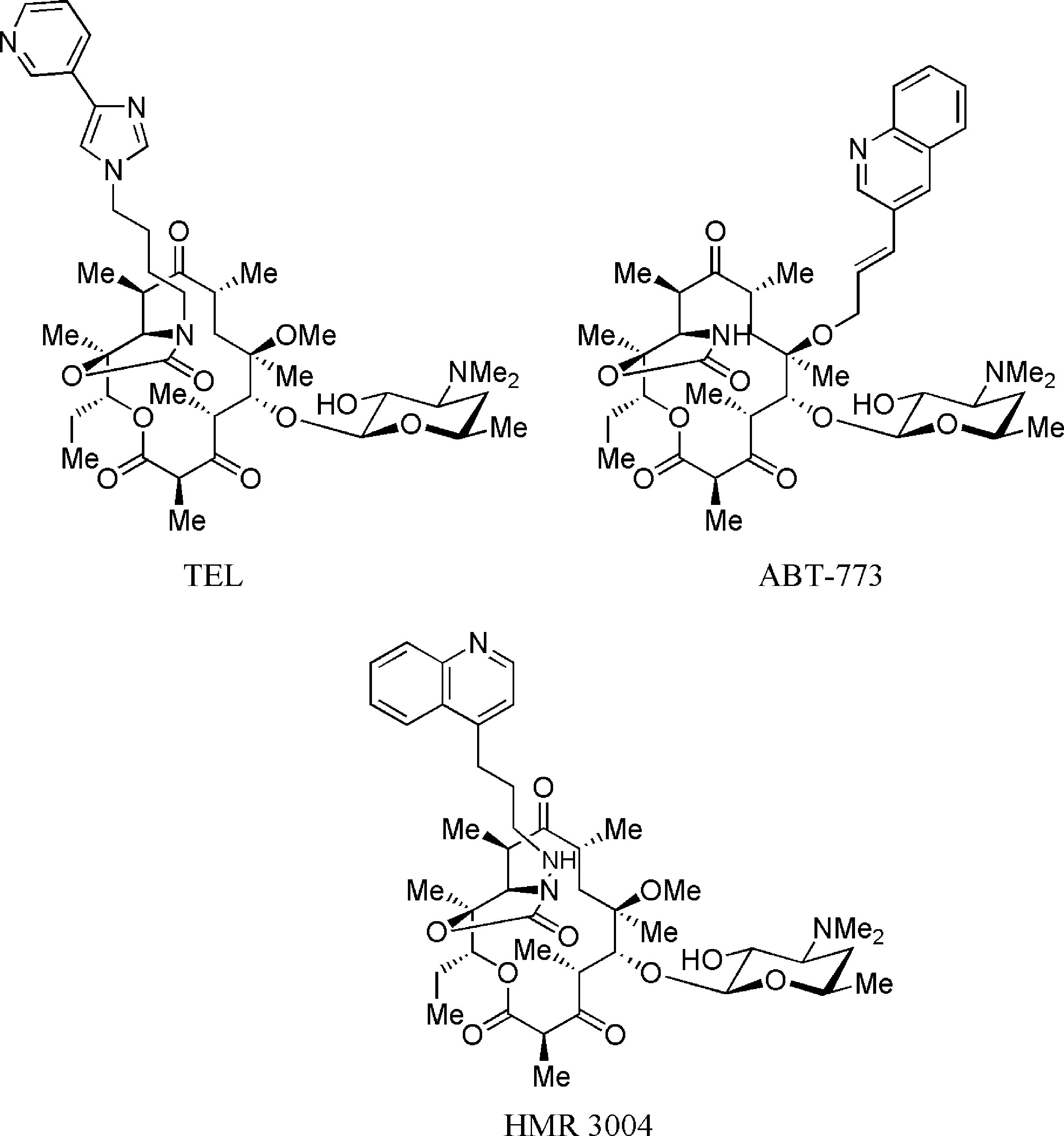
Structures of ketolides: Telithromycin, ABT-773, Hmr 3004.
CAS number: 1919888-06-4
LY3295668 is a complex, chiral, heterocyclic compound featuring a substituted piperidine core with two stereocenters at positions 2 and 4 in the R configuration. At position 1, it bears a 3-chloro-2-fluorobenzyl group, while the 4-position is substituted with a bulky side chain containing a fluorinated pyridine ring, which is further substituted with an amino-linked 5-methylpyrazole moiety. The piperidine ring also carries a carboxylic acid group at position 4 and a methyl group at position 2.
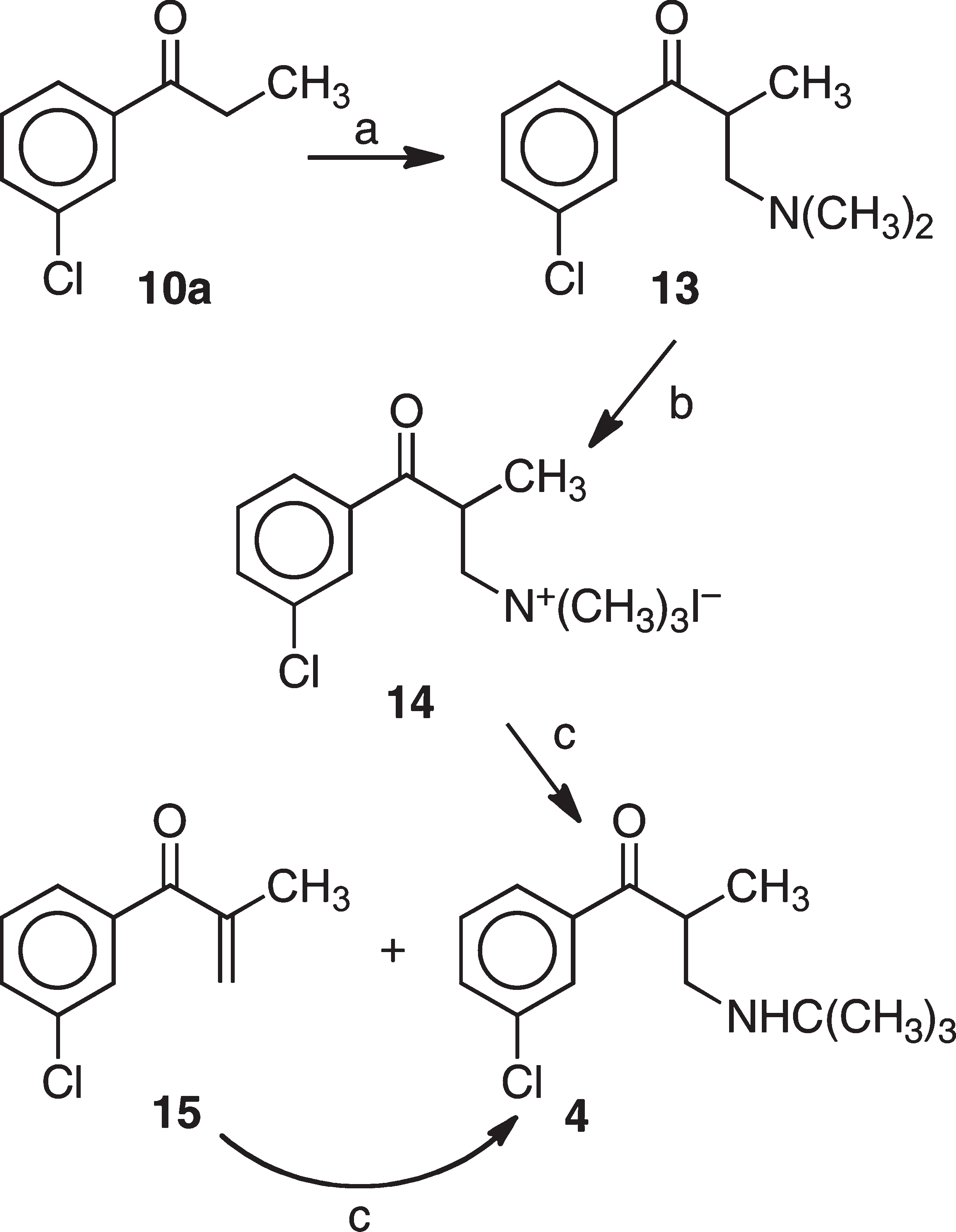
Scheme 3 shows the procedure used for the synthesis of 4. Subjection of 3'-chloropropiophenone (10a) to Mannich reaction conditions with aqueous formaldehyde and dimethylamine gave 13. The methiodide 14 was obtained by alkylation of 13 with iodomethane. Treatment of 14 with tert-butylamine gave first a mixture of 15 and the desired 4. Subjection of the mixture to excess tert-butylamine provided the desired target compound.
![Scheme 5 outlines the synthesis of 1a analogue 6. Treatment of 20 with hydrazine and potassium hydroxide in refluxing diethylene glycol (DEG) afforded 21. Compound 21 was cyclized to 22 using polyphosphoric acid (PPA). Subjection of 22 to acetamidobenzenesulfonyl azide (ABSA) in acetonitrile containing 1,4-diazabicyclo[5.4.0]undec-7-ene (DBU) yielded the diazo compound 23. Treatment of 23 with tert-butylamine in dry toluene in the presence of ruthenium acetate provided 6.](http://www.wlxkc.cn/picture/5213099_08.png)
Scheme 5 outlines the synthesis of 1a analogue 6. Treatment of 20 with hydrazine and potassium hydroxide in refluxing diethylene glycol (DEG) afforded 21. Compound 21 was cyclized to 22 using polyphosphoric acid (PPA). Subjection of 22 to acetamidobenzenesulfonyl azide (ABSA) in acetonitrile containing 1,4-diazabicyclo[5.4.0]undec-7-ene (DBU) yielded the diazo compound 23. Treatment of 23 with tert-butylamine in dry toluene in the presence of ruthenium acetate provided 6.
CAS number: 19216-56-9
Prazosin is a drug used to treat hypertension. Prazosin is marketed by Pfizer and was initially approved by the FDA in 1988. It belongs to the class of drugs known as alpha-1 antagonists. Recently, many studies have evaluated the benefits of this drug in controlling the symptoms of post-traumatic stress disorder (PTSD) and associated nightmares.
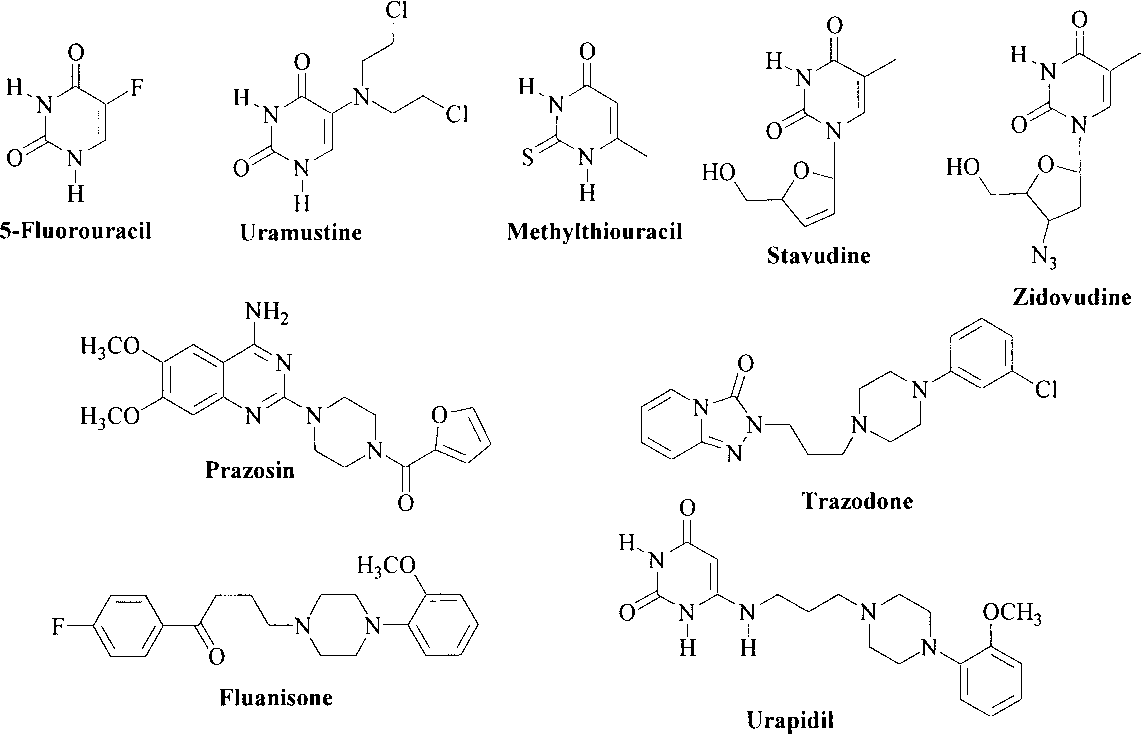
Several Pharmacologically Useful Agents Containing Uracil and Piperazine Moieties: 5-Fluorouracil, Uramustine, Methylthiouracil, stavudine, zidovudine, prazosin, trazodone, Fluanisone, urapidil.
CAS number: 1923-70-2
Tetrabutylammonium perchlorate is a high-solubility quaternary ammonium salt ideal for organic synthesis and electrochemistry. It has been synthesized from precipitated silver halides and drying agents.
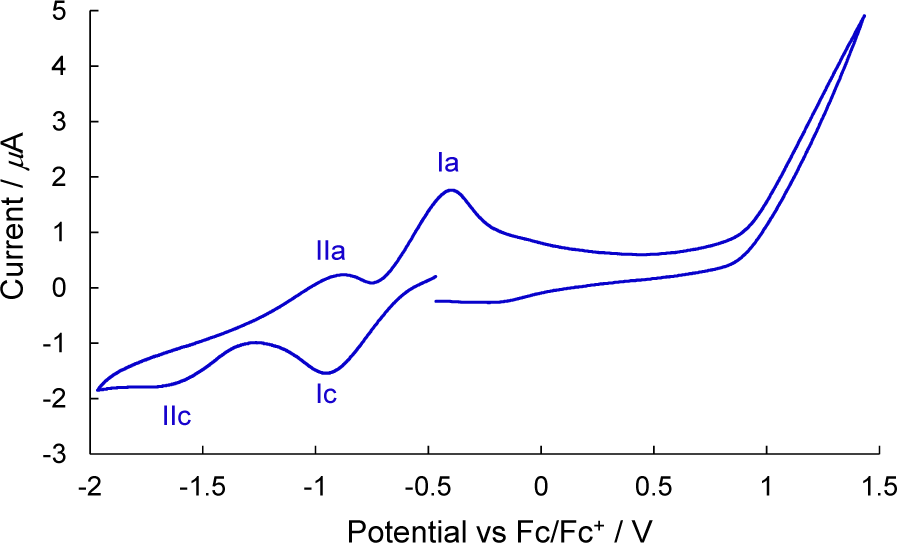
Cyclic voltammogram of 3 measured in THF containing tetrabutylammonium perchlorate (0.1 M) as a supporting electrolyte. AgQRE was used as a reference electrode. Platinum wire was used as the working and counter electrodes. The scan rate was 50 mV s−1. Fc/Fc+ was used as external reference.