Chemicals list & Research Gallery
CAS number: 183675-82-3
1-methyl-N-[2-(4-methylpentan-2-yl)-3-thienyl]-3-(trifluoromethyl)pyrazole-4-carboxamide is an aromatic amide obtained by formal condensation of the carboxy group of 1-methyl-3-(trifluoromethyl)pyrazole-4-carboxylic acid with the amino group of 2-(4-methylpentan-2-yl)thiophen-3-amine. It is an aromatic amide, an organofluorine compound, a member of pyrazoles and a member of thiophenes.
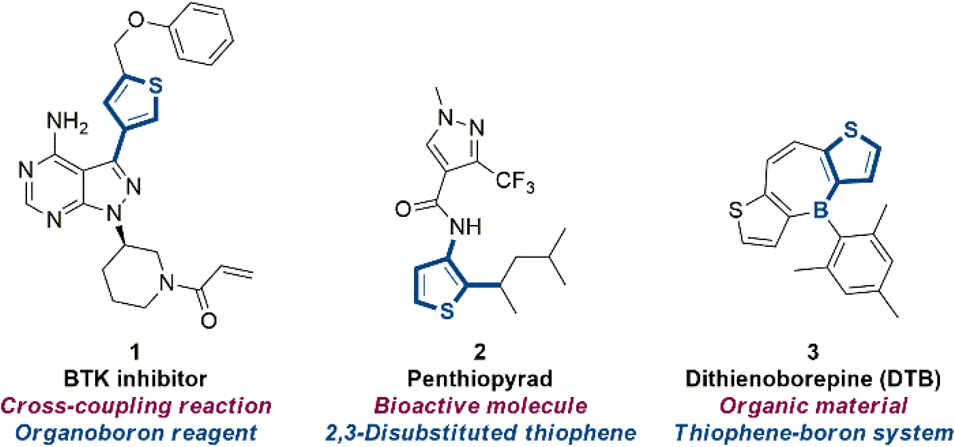
Synthetic importance of thiophene building blocks.
CAS number: 1839-72-1
2-Phenylbenzofuran is a substituted benzofuran, meaning it has a benzofuran core structure with a phenyl group attached to the second carbon position.
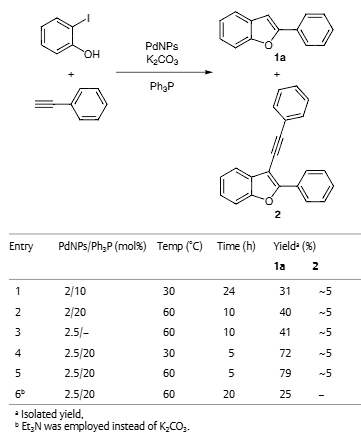
Optimization of the Reaction Conditions for the Synthesis of 2-Phenylbenzofuran (1a)
CAS number: 1841-19-6
A long-acting injectable antipsychotic agent used for chronic schizophrenia.
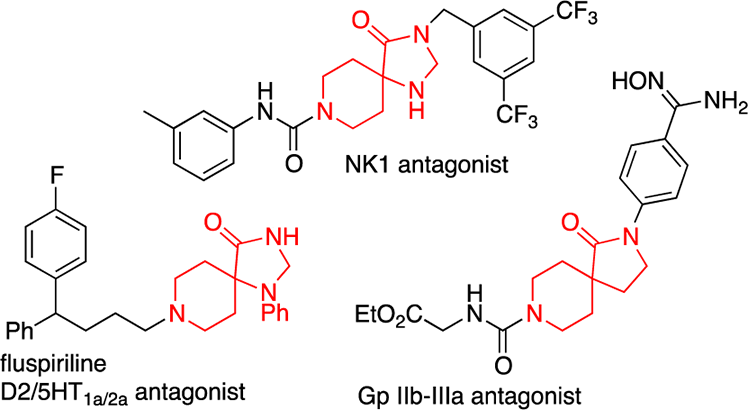
A selection of bioactive spirocyclic piperidines.
CAS number: 184475-35-2
Gefitinib (originally coded ZD1839) is a drug used in the treatment of certain types of cancer. Acting in a similar manner to erlotinib (marketed as Tarceva), gefitinib selectively targets the mutant proteins in malignant cells. It is marketed by AstraZeneca under the trade name Iressa.
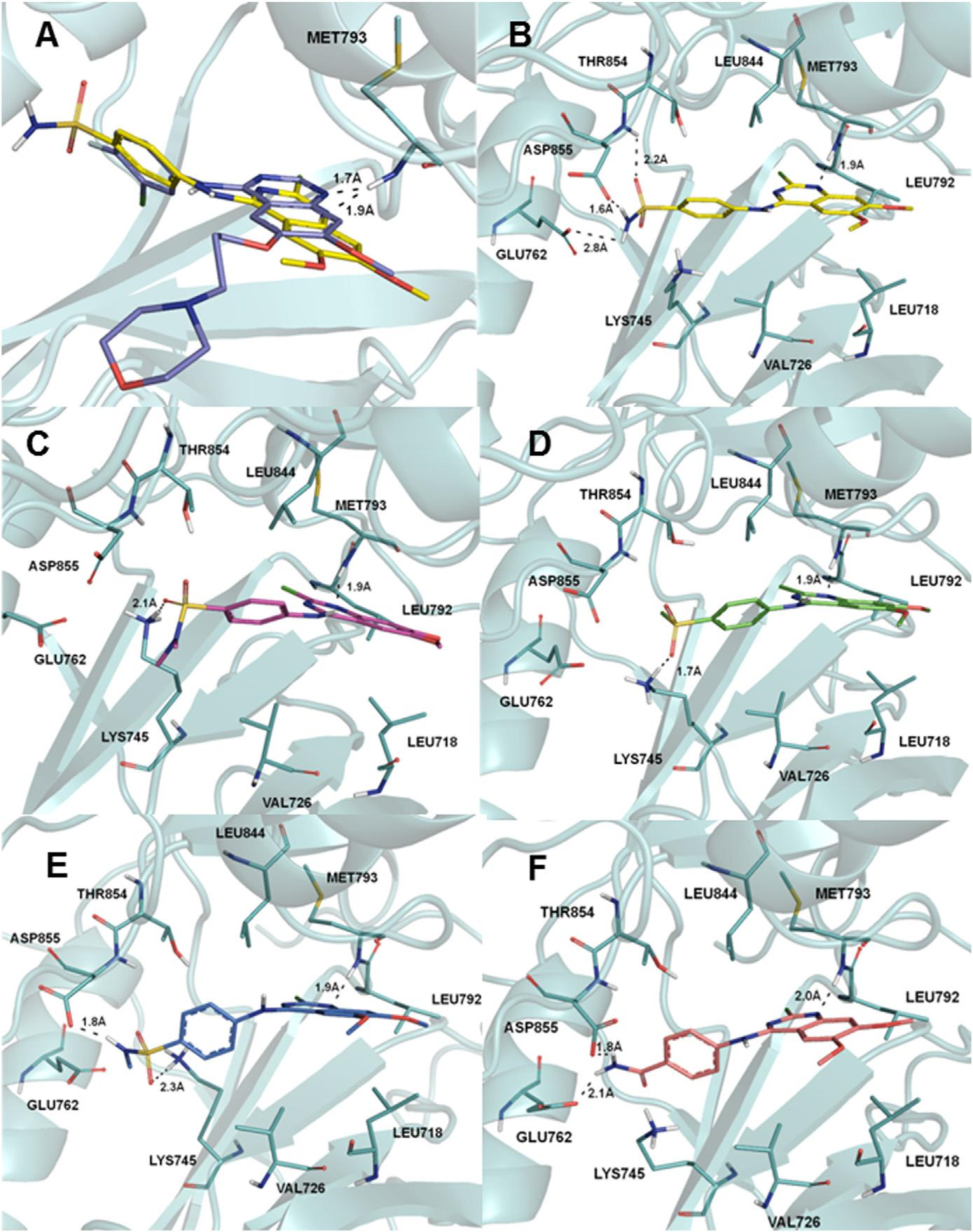
(A) Superposition of 8g (yellow) and gefitinib (2, purple) poses inside the EGFR tyrosine kinase ATP binding site. (B) Binding interactions of 8g (yellow) with EGFRwt. (C) Binding interactions of 8b (magenta) with EGFRwt. (D) Binding interactions of 8e (green) with EGFRwt. (E) Binding interactions of 8k (blue) with EGFRwt. (F) Binding interactions of 8o (light pink) with EGFRwt.
CAS number: 18483-17-5
Gallotannin is a class of hydrolysable tannins obtained by condensation of the carboxy group of gallic acid (and its polymeric derivatives) with the hydroxy groups of a monosaccharide (most commonly glucose). It is functionally related to a gallic acid.
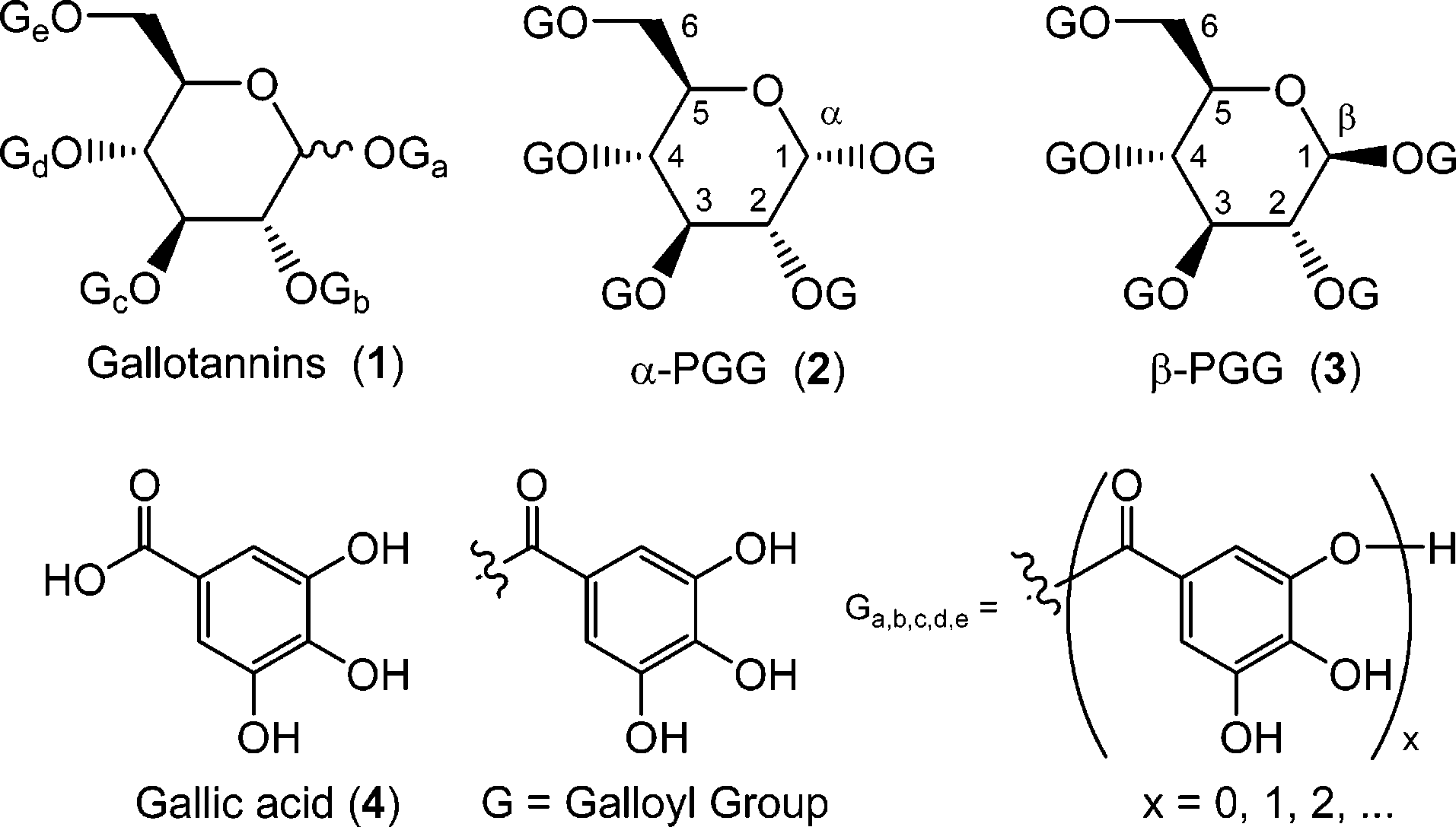
Structure of gallotannins and the two anomers of PGG, 2 and 3.
CAS number: 18542-45-5
1-(2-Propynyloxy)naphthalene is an organic compound. It is a liquid, colorless substance. It is also known as 1-prop-2-ynoxynaphthalene or 1-(2-propyn-1-yloxy)naphthalene.
![Propargyl ethers 7-chloro-(4-propargyloxy)quinoline, 1-propargyloxynaphthalene, and 2-propargyloxybenzophenone reacted with [AuCl(PPh3)] in the presence of KOH to produce colorless, air- and humidity-stable gold(I) alkynyl complexes ≡[Au(C COCH2Ar)(PPh3)] (1-3) in good yields (Scheme 1).](http://www.wlxkc.cn/picture/4919175_04.png)
Propargyl ethers 7-chloro-(4-propargyloxy)quinoline, 1-propargyloxynaphthalene, and 2-propargyloxybenzophenone reacted with [AuCl(PPh3)] in the presence of KOH to produce colorless, air- and humidity-stable gold(I) alkynyl complexes ≡[Au(C COCH2Ar)(PPh3)] (1-3) in good yields (Scheme 1).
CAS number: 186424-51-1
Cerasonine is a natural product that has been isolated from the stem bark of the plant Polyalthia cerasoides. It belongs to a class of compounds called oxoprotoberberine alkaloids.
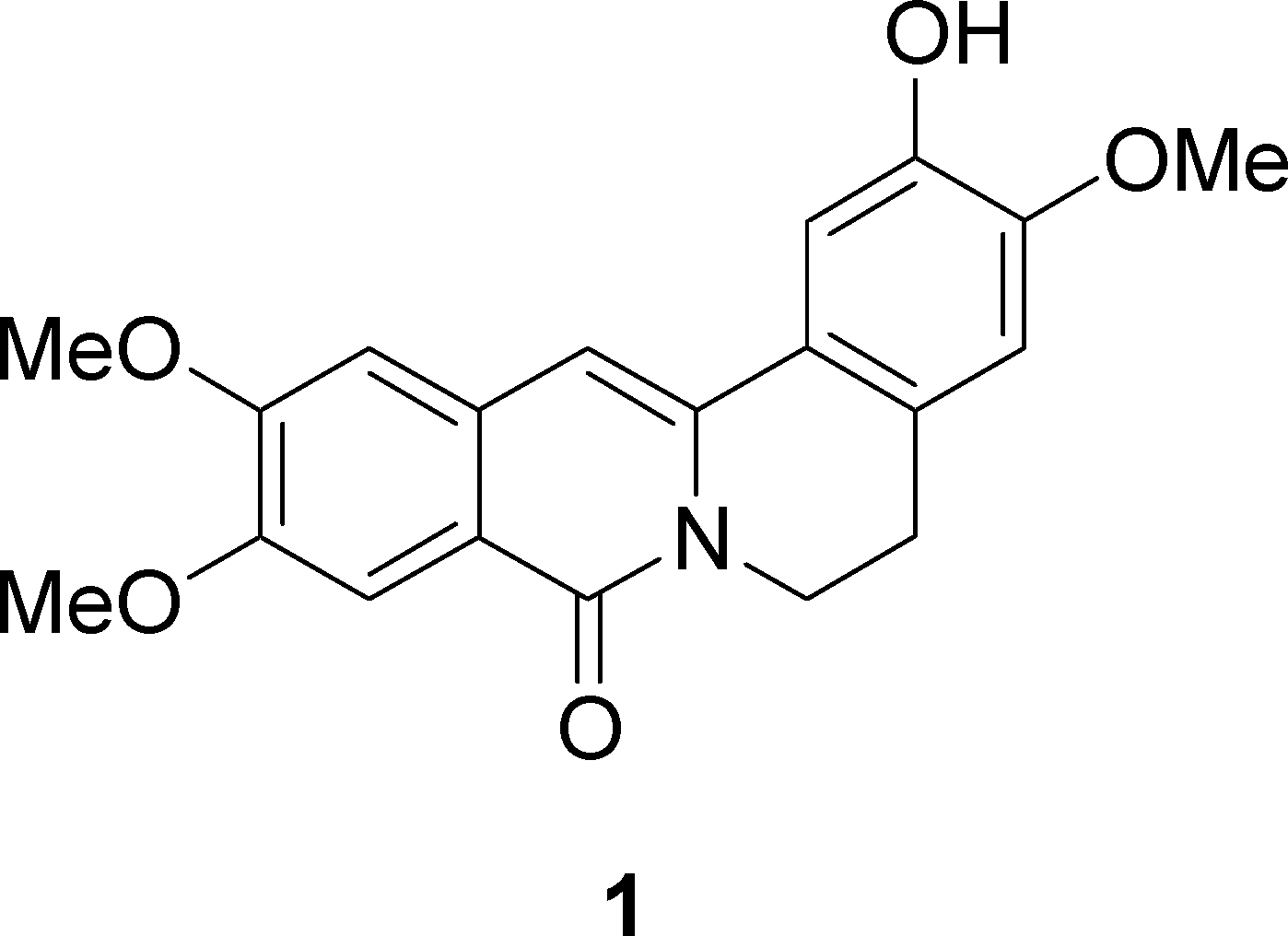
Structure of the Protoberberine Alkaloid, Cerasonine
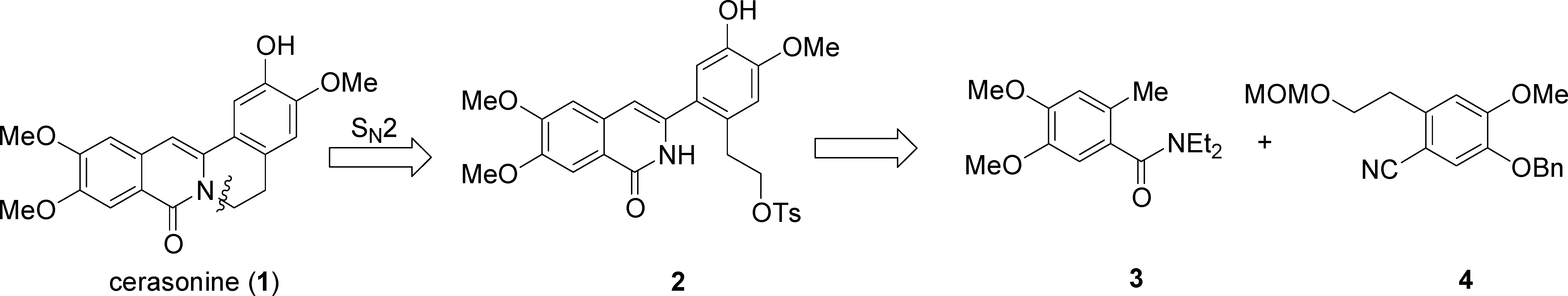
Retrosynthetic Analysis of Cerasonine
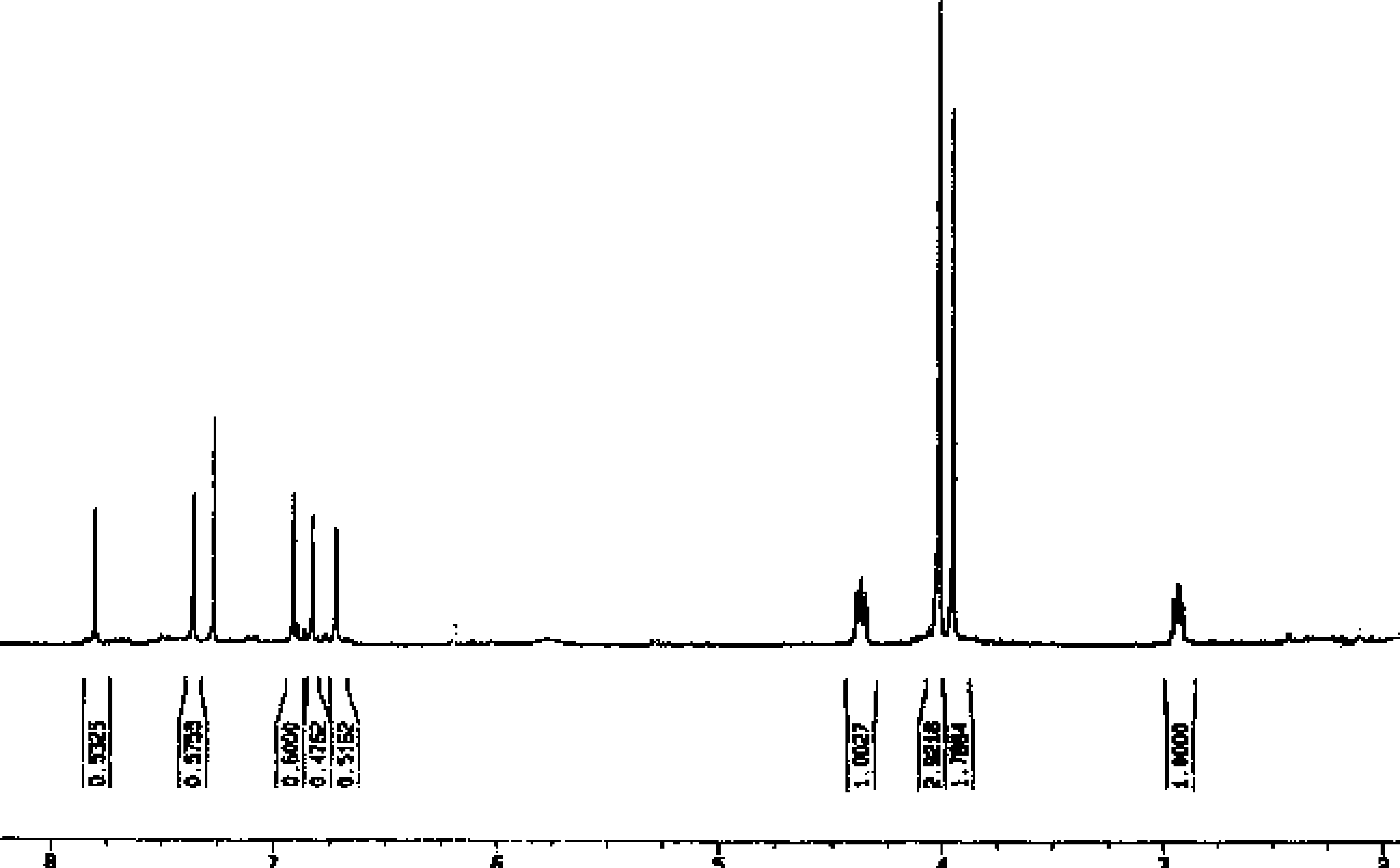
H-NMR Spectra of Synthetic Cerasonine
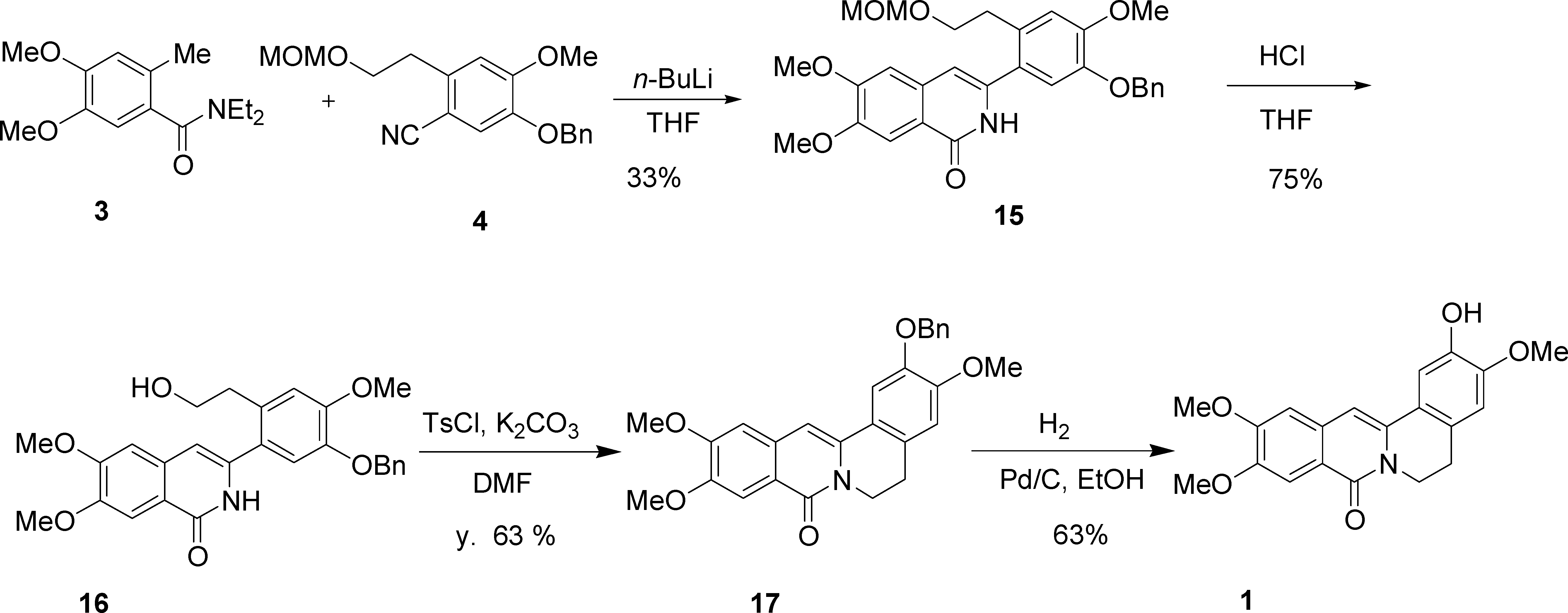
Synthesis of Cerasonine
CAS number: 186953-55-9
An amidoxime is an organic chemical compound containing both a hydroxyimino (═NOH) and an amino (—NH₂) group attached to the same carbon atom. Its general formula is R-C(NH₂)═NOH. Amidoximes are important versatile building blocks in organic synthesis, used to create other complex molecules, particularly heterocycles. They are also valued for their ability to chelate (bind) metal ions, making them useful in processes like extracting trace metals such as uranium from seawater and as adsorbents for heavy metal pollutants from water.
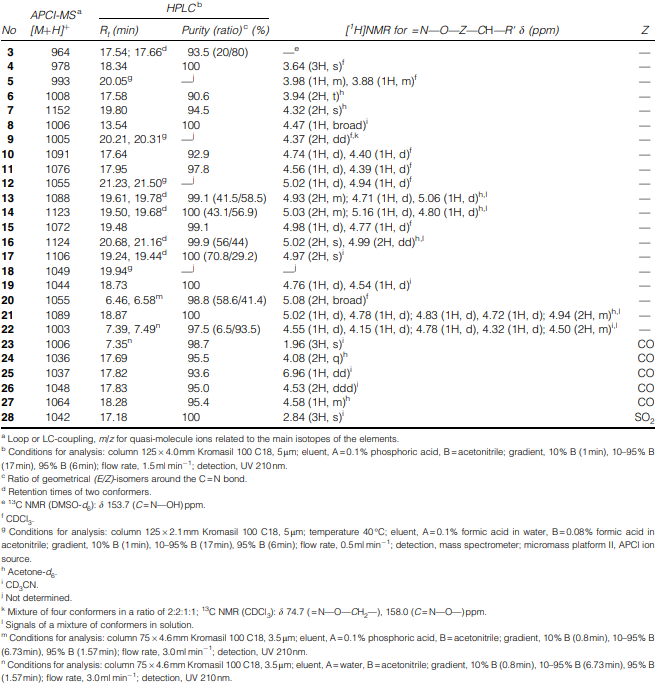
Analytical and spectroscopic data of the N-methylated amidoxime analogues of PF1022A (I)
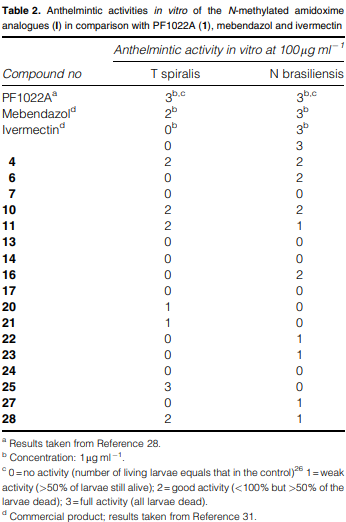
Anthelmintic activities in vitro of the N-methylated amidoxime analogues (I) in comparison with PF1022A (1), mebendazol and ivermectin
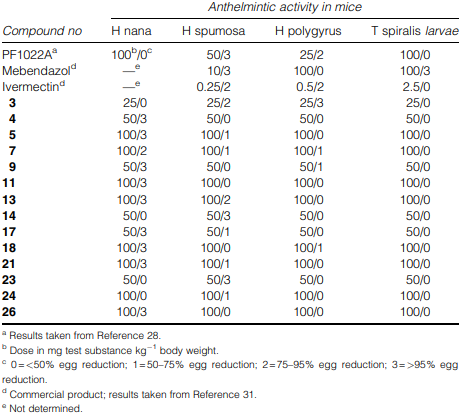
Anthelmintic activities of the N-methylated amidoxime analogues (I) in comparison with PF1022A (1), mebendazol and ivermectin
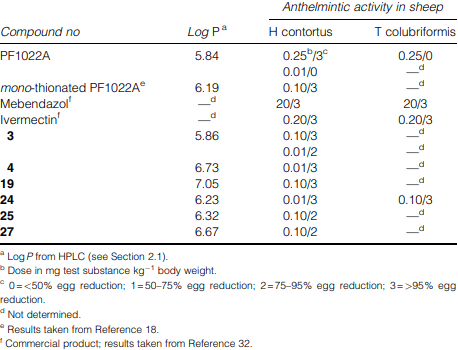
Anthelmintic activities and lipophilicities of the N-methylated amidoxime analogues (I) in comparison with PF1022A (1), mono-thionated PF1022A (2), mebendazol and ivermectin
CAS number: 187235-37-6
PA-824 is a novel antibacterial agent that has shown in vitro activity against both drug-sensitive and drug-resistant Mycobacterium tuberculosis.
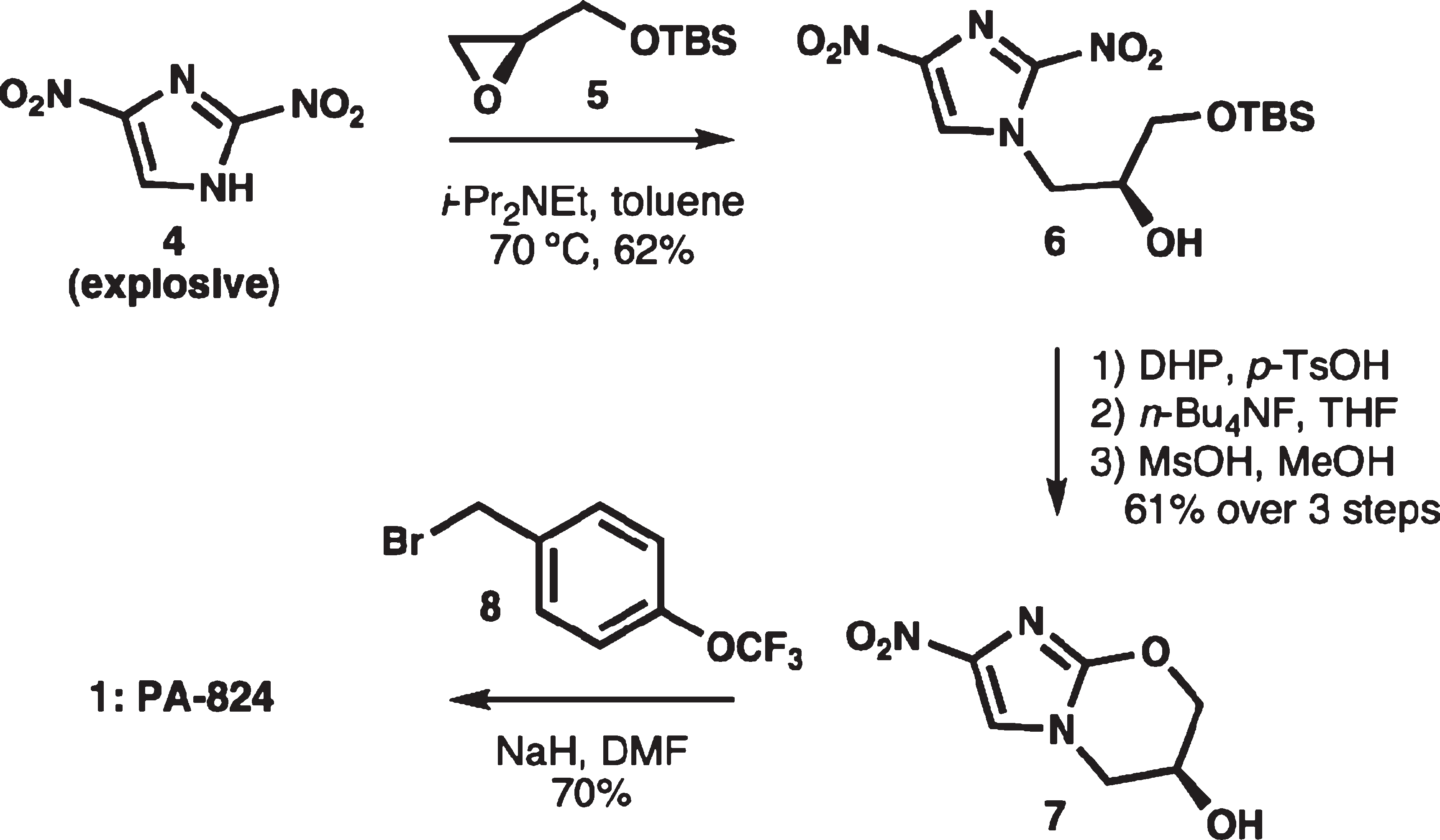
Original production process of PA-824
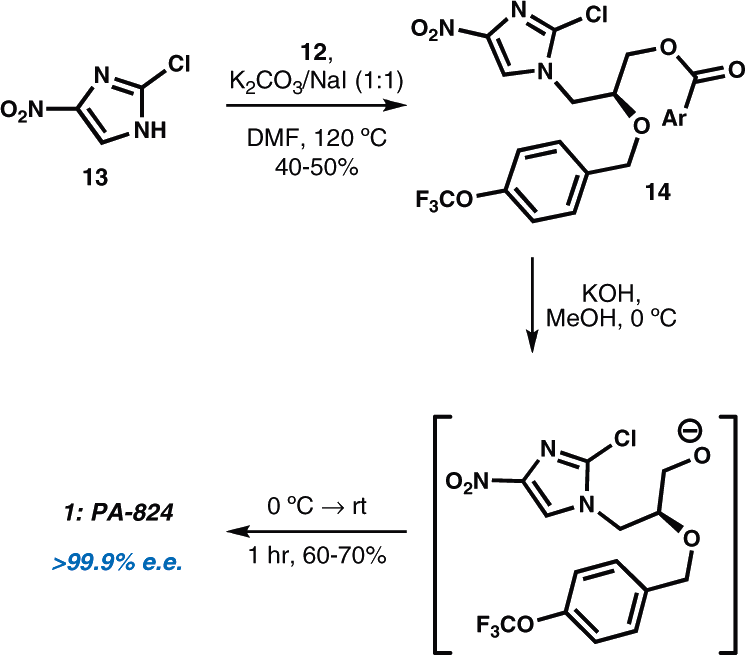
Synthesis of PA-824
CAS number: 1873-77-4
Tris(trimethylsilyl)silane is used in hydrosilylations of carbonyls, radical reactions, reductions of acid chlorides, and carbon-halogen bonds.
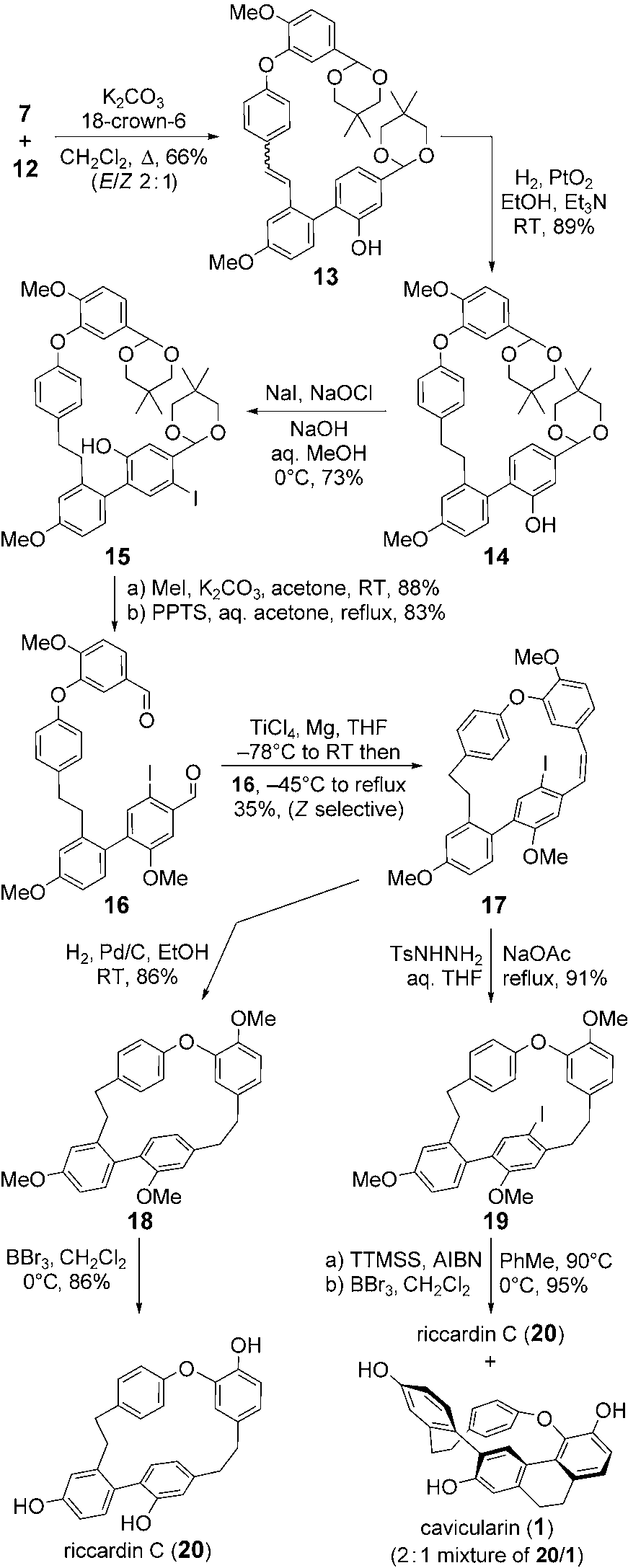
Total synthesis of cavicularin (1) and riccardin C (20). Ts =p-toluenesulfonyl, TTMSS=tris(trimethylsilyl)silane, AIBN=azo-bis(isobutyronitrile).