LY3295668
CAS number: 1919888-06-4
LY3295668 is a complex, chiral, heterocyclic compound featuring a substituted piperidine core with two stereocenters at positions 2 and 4 in the R configuration. At position 1, it bears a 3-chloro-2-fluorobenzyl group, while the 4-position is substituted with a bulky side chain containing a fluorinated pyridine ring, which is further substituted with an amino-linked 5-methylpyrazole moiety. The piperidine ring also carries a carboxylic acid group at position 4 and a methyl group at position 2.
Related images
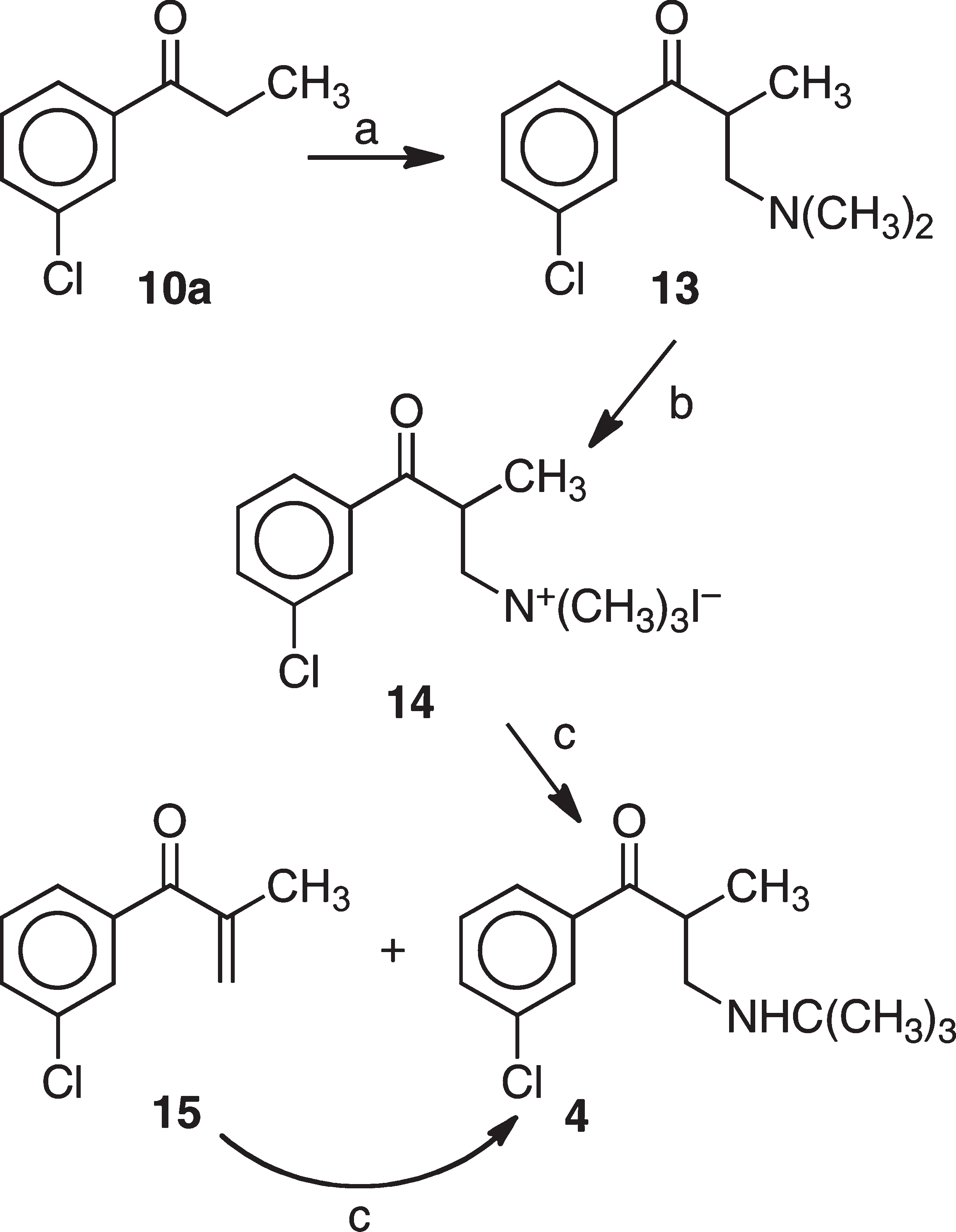
Scheme 3 shows the procedure used for the synthesis of 4. Subjection of 3'-chloropropiophenone (10a) to Mannich reaction conditions with aqueous formaldehyde and dimethylamine gave 13. The methiodide 14 was obtained by alkylation of 13 with iodomethane. Treatment of 14 with tert-butylamine gave first a mixture of 15 and the desired 4. Subjection of the mixture to excess tert-butylamine provided the desired target compound.
![Scheme 5 outlines the synthesis of 1a analogue 6. Treatment of 20 with hydrazine and potassium hydroxide in refluxing diethylene glycol (DEG) afforded 21. Compound 21 was cyclized to 22 using polyphosphoric acid (PPA). Subjection of 22 to acetamidobenzenesulfonyl azide (ABSA) in acetonitrile containing 1,4-diazabicyclo[5.4.0]undec-7-ene (DBU) yielded the diazo compound 23. Treatment of 23 with tert-butylamine in dry toluene in the presence of ruthenium acetate provided 6.](http://www.wlxkc.cn/picture/5213099_08.png)
Scheme 5 outlines the synthesis of 1a analogue 6. Treatment of 20 with hydrazine and potassium hydroxide in refluxing diethylene glycol (DEG) afforded 21. Compound 21 was cyclized to 22 using polyphosphoric acid (PPA). Subjection of 22 to acetamidobenzenesulfonyl azide (ABSA) in acetonitrile containing 1,4-diazabicyclo[5.4.0]undec-7-ene (DBU) yielded the diazo compound 23. Treatment of 23 with tert-butylamine in dry toluene in the presence of ruthenium acetate provided 6.
Related Questions and Answers
No related questions yet