Chemicals list & Research Gallery
CAS number: 68332-96-7
Burkholderia cepacia is a plant phytogen and is known as a hardy and versatile organism.
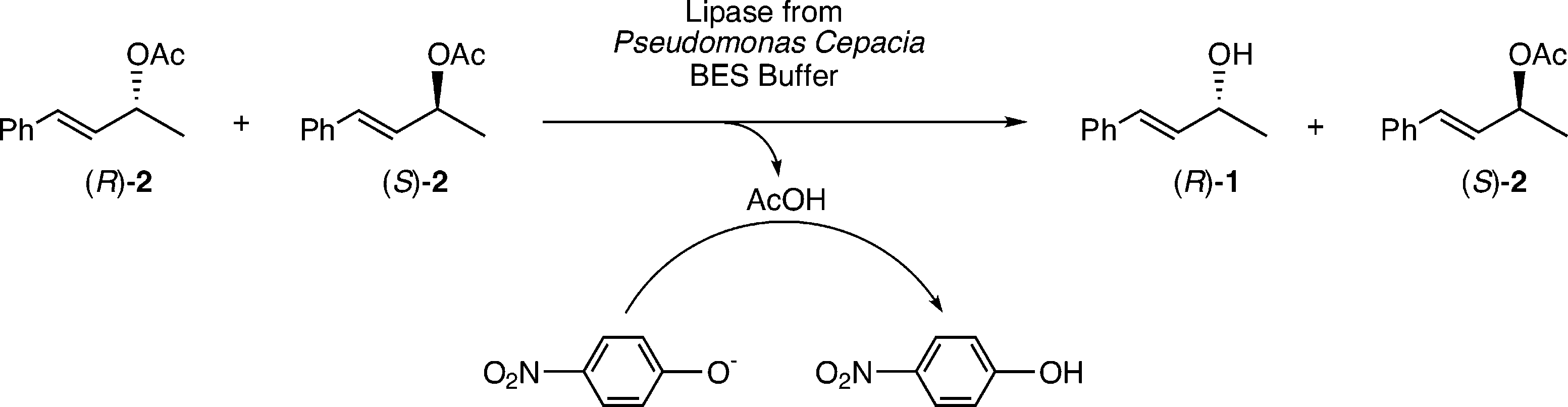
EMDee assay of allylic acetate 2 using the lipase from Pseudomonas cepacia.
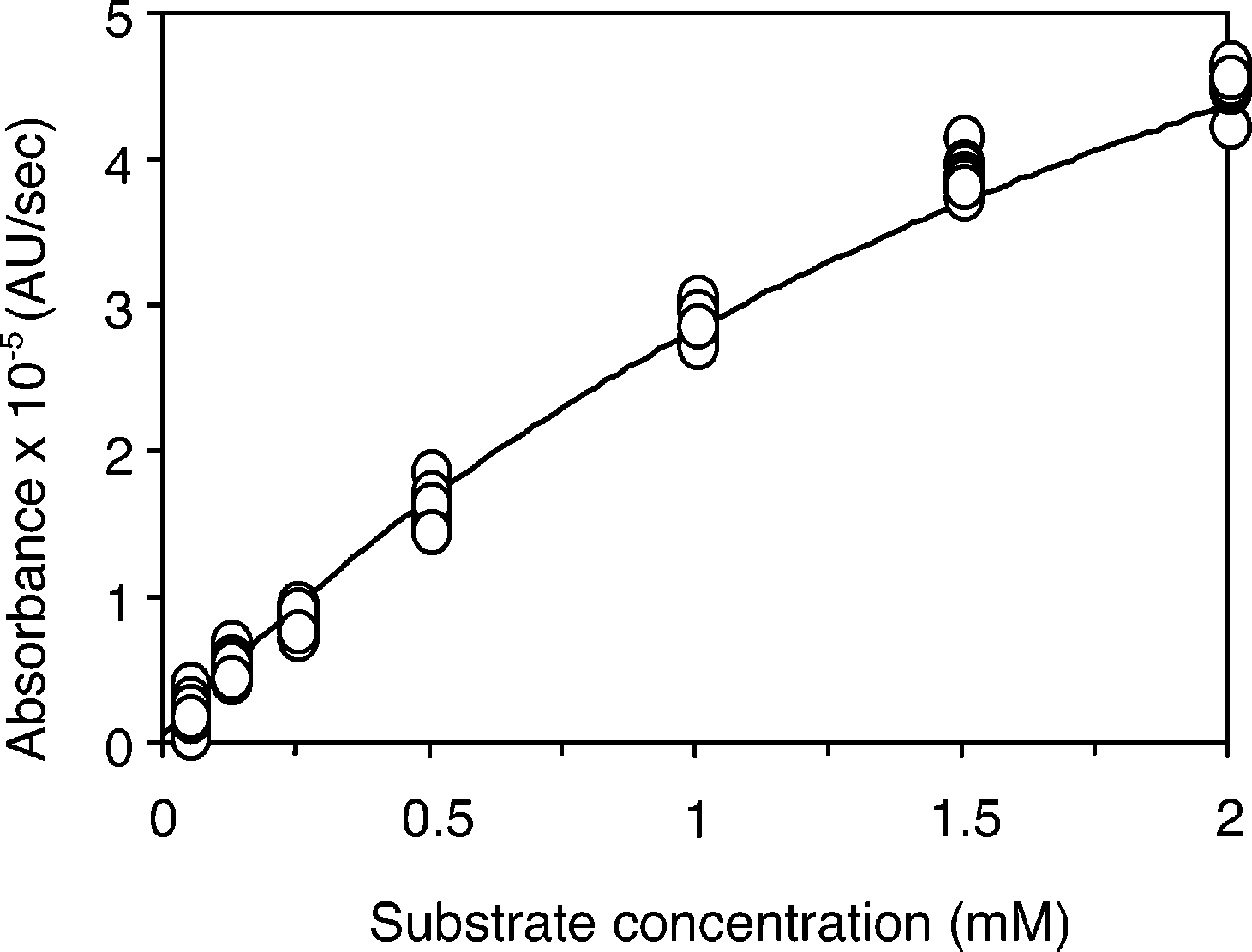
Plot of the rate of the lipase-catalyzed reaction as a function of the concentration of (R)-2.
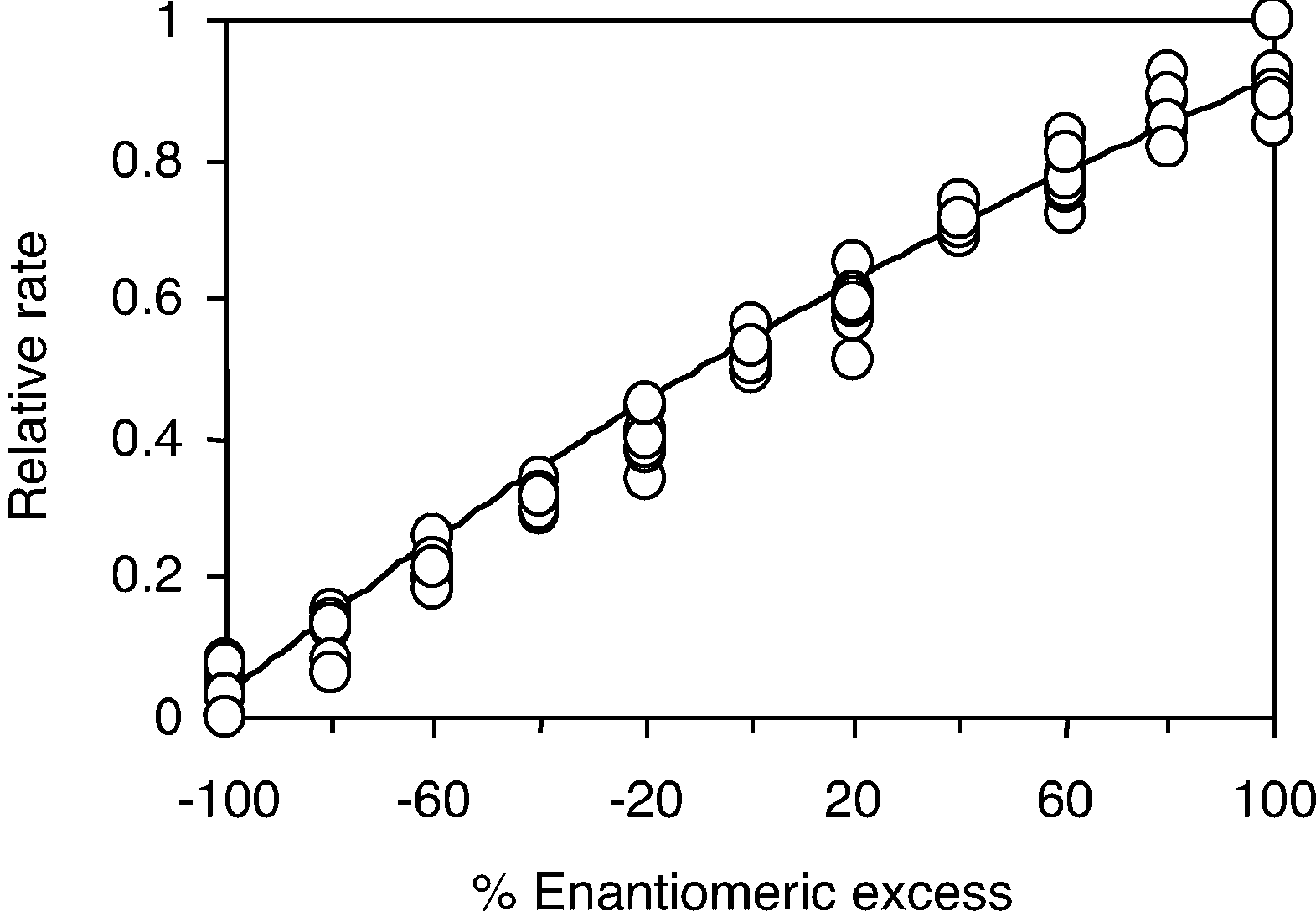
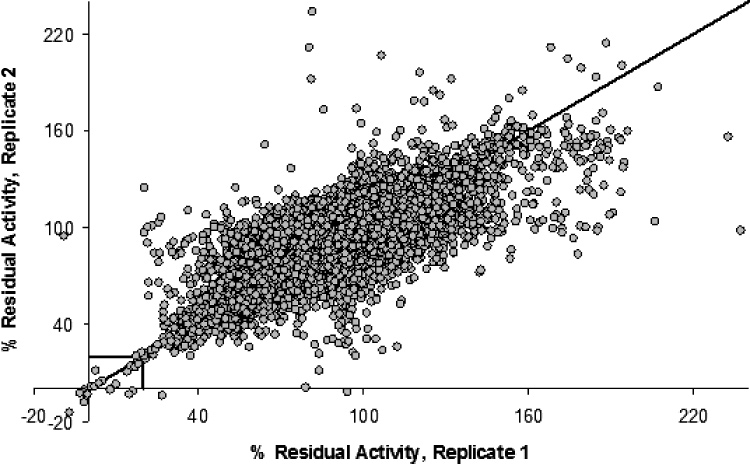
Analysis of 88 samples of compound 2 that range in ee from 100% (S) to 100% (R) using the lipase from Pseudomonas cepacia.
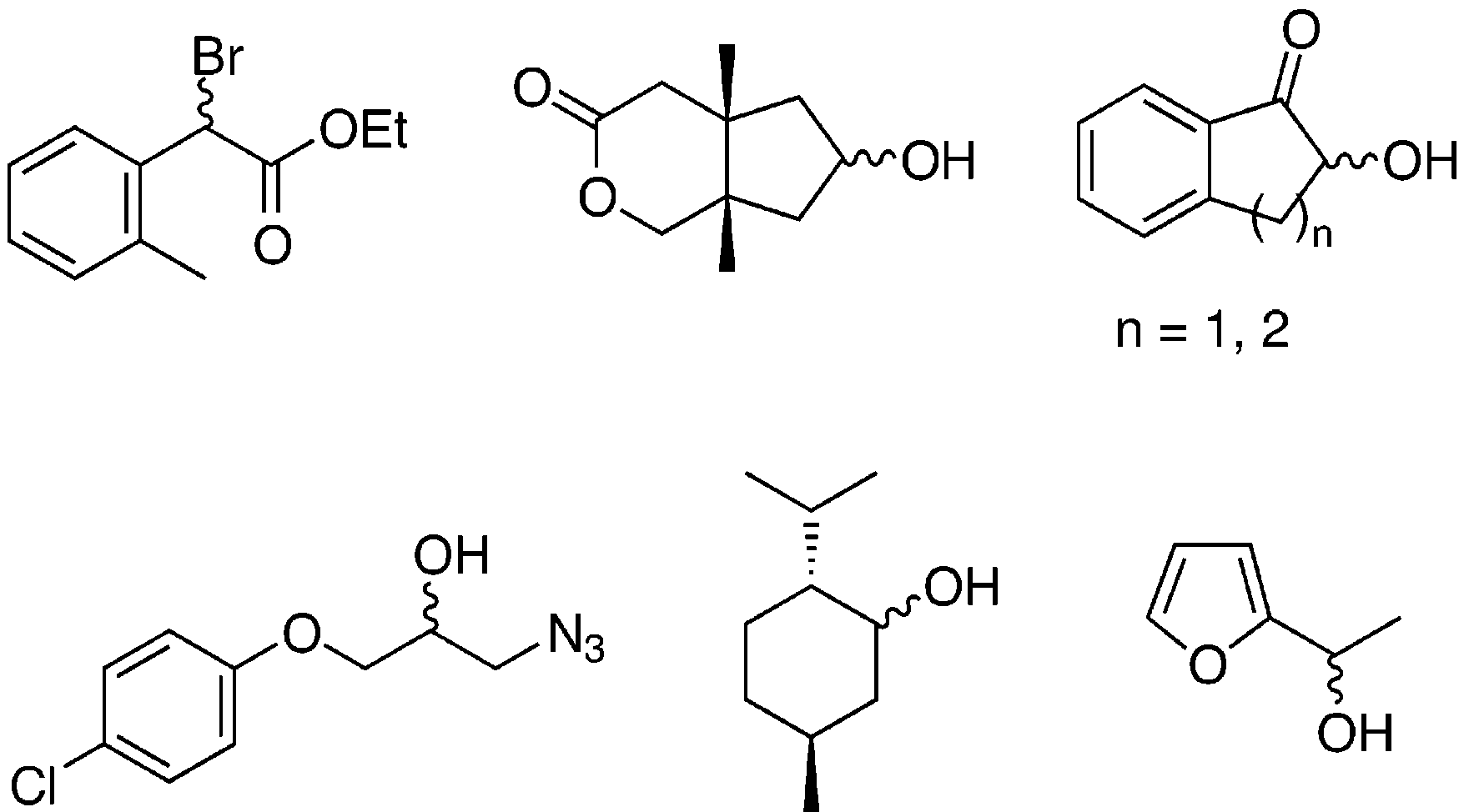
Structures of several substrates for the lipase from Pseudomonas cepacia.
CAS number: 68475-71-8
Carboxylic acids, C2–3 is a low-molecular-weight carboxylic acids containing two to three carbon atoms, primarily including acetic acid (C2) and propionic acid (C3).
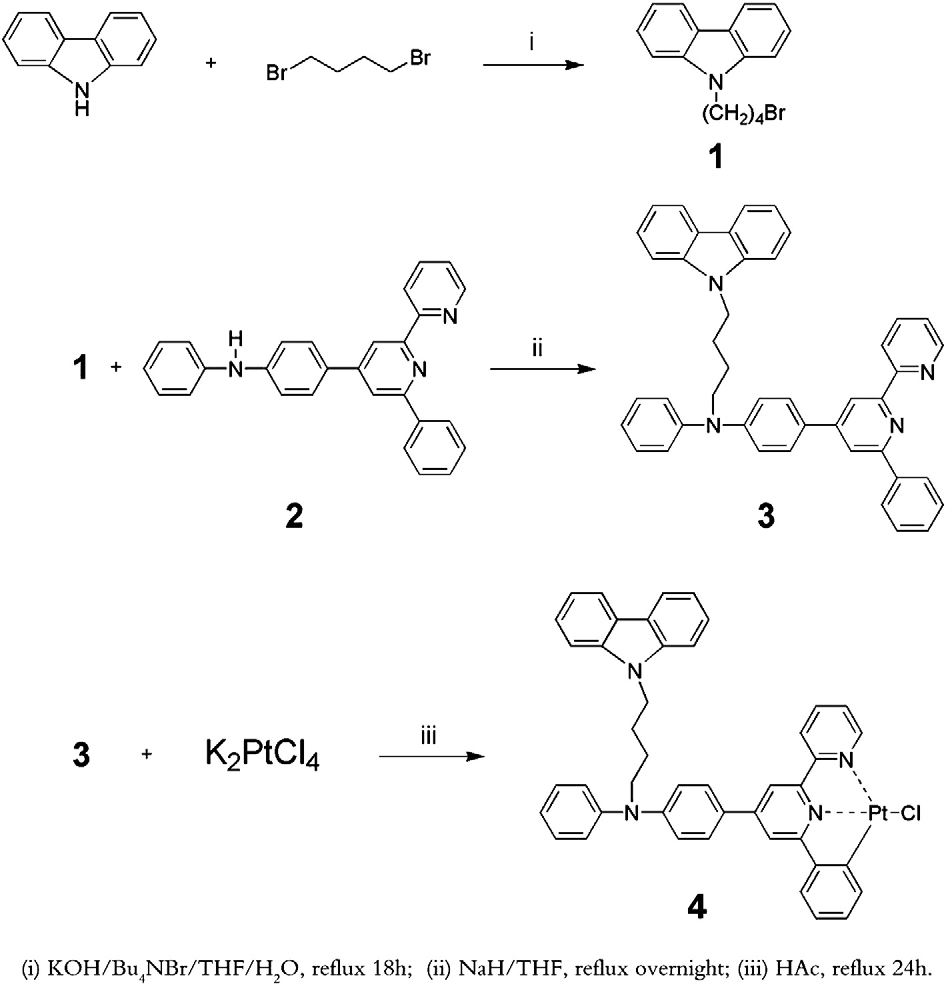
Carbazole was directly coupled with excess 1,4-dibromobutane under strong alkaline conditions to give 9-(4-bromobutane)carbazole (1). 4-(p-bromophenyl)-6-phenyl-2,2′-bipyridine was synthesized by the Kröhnke method. A strong palladium-catalyzed Buchwald method and an efficient Pd(OAc)2/DPEphos catalyst/ligand system were used to promote the condensation of aniline with 4-(p-bromophenyl)-6-phenyl-2,2′-bipyridine. The resulting intermediate (2) was then reacted with 9-(4-bromobutane)carbazole (1) to give the ligand HL (3). The ligand HL (3) and K2PtCl4 were refluxed in glacial acetic acid for 24 h to synthesize the cyclometalated Pt(II) chloride 4 with a yield of more than 80%.
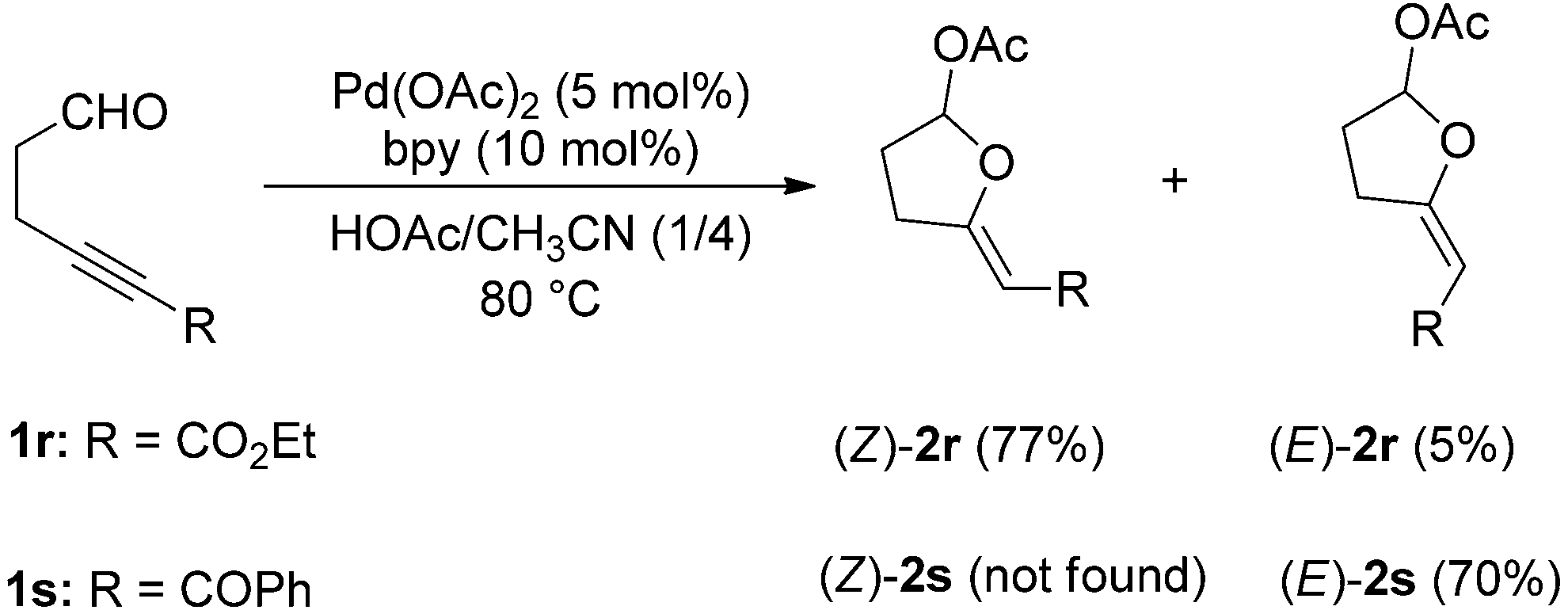
The reactions of substrates 1r and 1s with acetic acid.
![Synthesis route of HL3 and the corresponding cyclometalated PtII complex [Pt(L3)Cl] ACHTUNRTGNEUNG[PF6]: a) NaH, 1,5-dibromopentane; b) PPh3; c) K2PtCl4, glacial acetic acid.](http://www.wlxkc.cn/picture/4465522_05.png)
Synthesis route of HL3 and the corresponding cyclometalated PtII complex [Pt(L3)Cl] ACHTUNRTGNEUNG[PF6]: a) NaH, 1,5-dibromopentane; b) PPh3; c) K2PtCl4, glacial acetic acid.
CAS number: 68583-22-2
A species of gram-negative, facultatively anaerobic, rod-shaped bacteria (GRAM-NEGATIVE FACULTATIVELY ANAEROBIC RODS) commonly found in the lower part of the intestine of warm-blooded animals. It is usually nonpathogenic, but some strains are known to produce DIARRHEA and pyogenic infections. Pathogenic strains (virotypes) are classified by their specific pathogenic mechanisms such as toxins (ENTEROTOXIGENIC ESCHERICHIA COLI), etc.
Results of the high-throughput screen of E. coli CBL vs 50 000 small molecules.
CAS number: 68583-24-4
Klebsiella pneumoniae is a Gram-negative, non-motile, encapsulated, lactose-fermenting, facultative anaerobic, rod-shaped bacterium. It appears as a mucoid lactose fermenter on MacConkey agar.
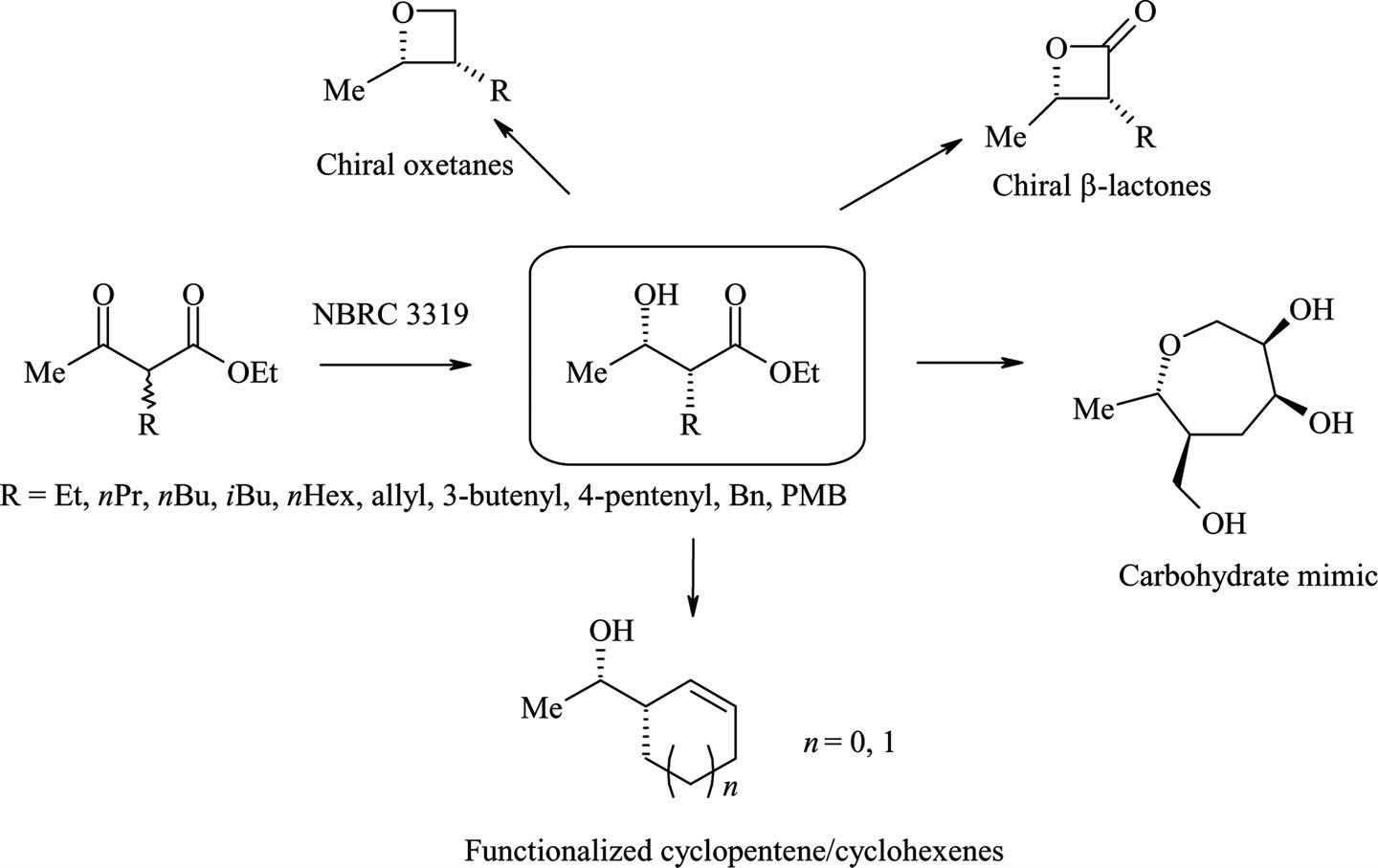
Klebsiella pneumoniae (NBRC 3319) mediated synthesis of various 2-substitited β-hydroxy esters and proposed synthetic manipulations.
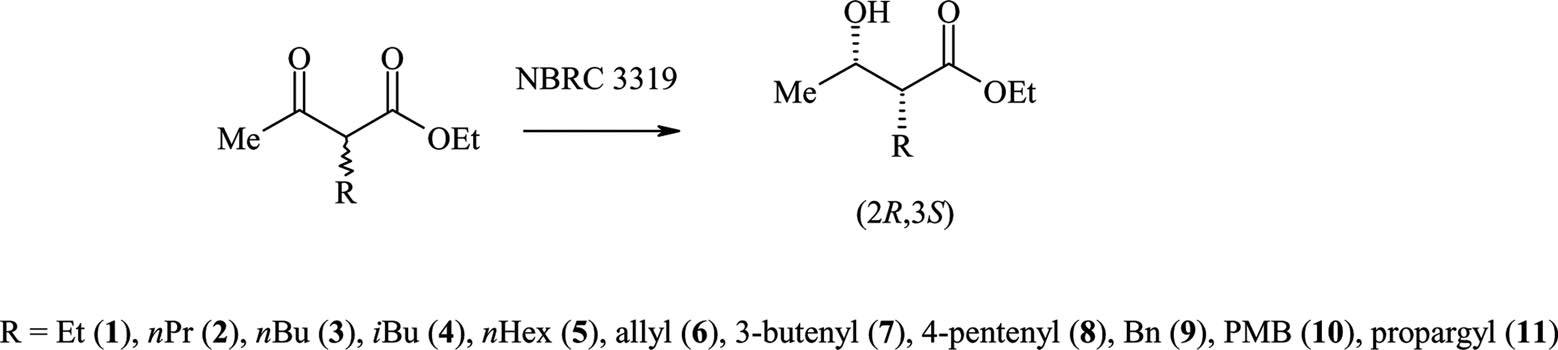
Exclusive formation of syn-β-hydroxy oxo esters by Klebsiella pneumoniae.
CAS number: 68583-32-4
Pseudomonas syringae strain ESC-10 is a naturally occurring bacterium, originally isolated from apples, that is used as a biological control agent to prevent fungal diseases on fruits.
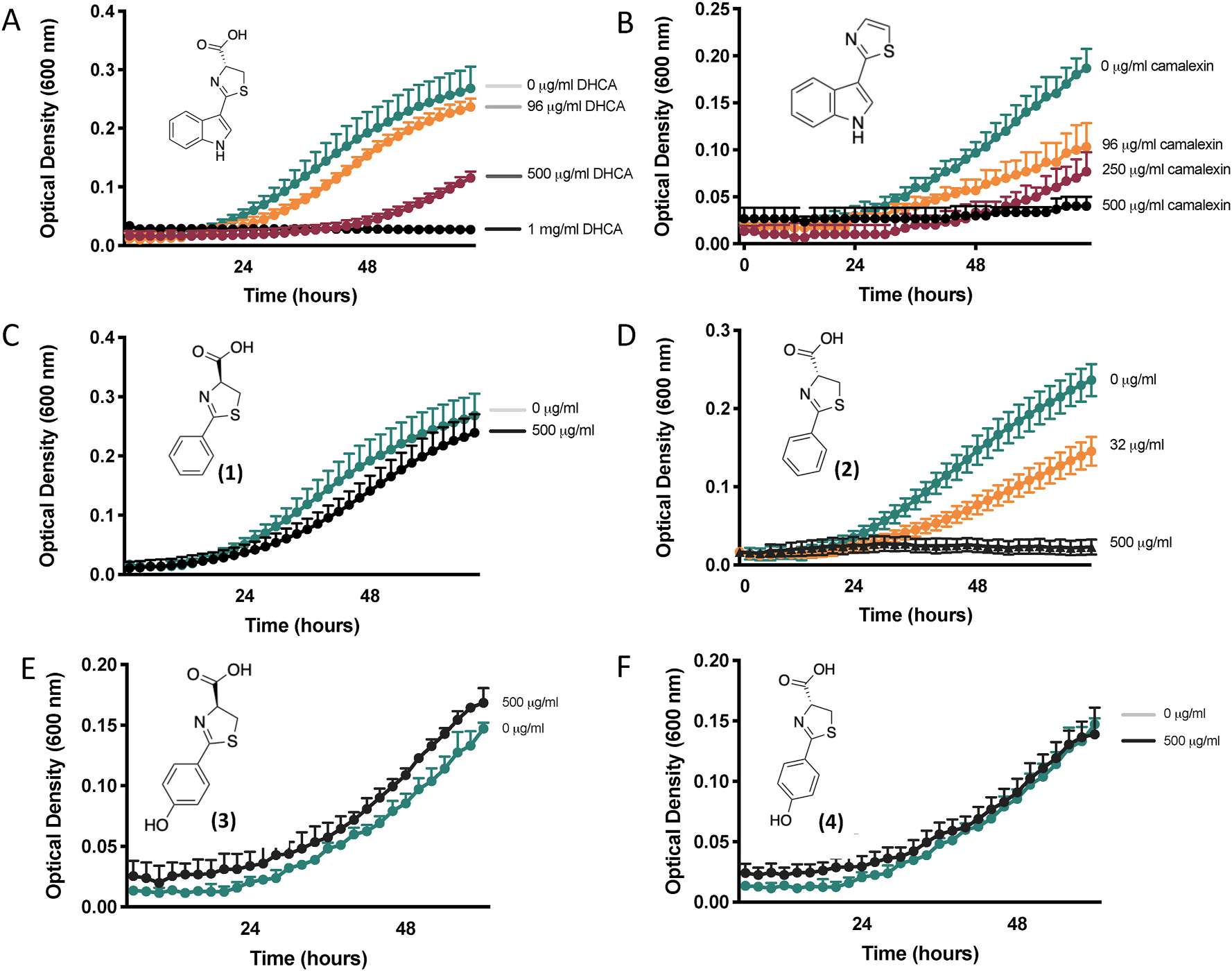
In vitro growth of Pseudomonas syringae (Pst) in the presence of dihydrocarbazine acid (DHCA), carbazine acid, or DHCA analogs
Effects of dihydrocarbamazepine (DHCA) and salicylic acid (SA) on biofilm formation of Pseudomonas syringae (Pst) in vitro
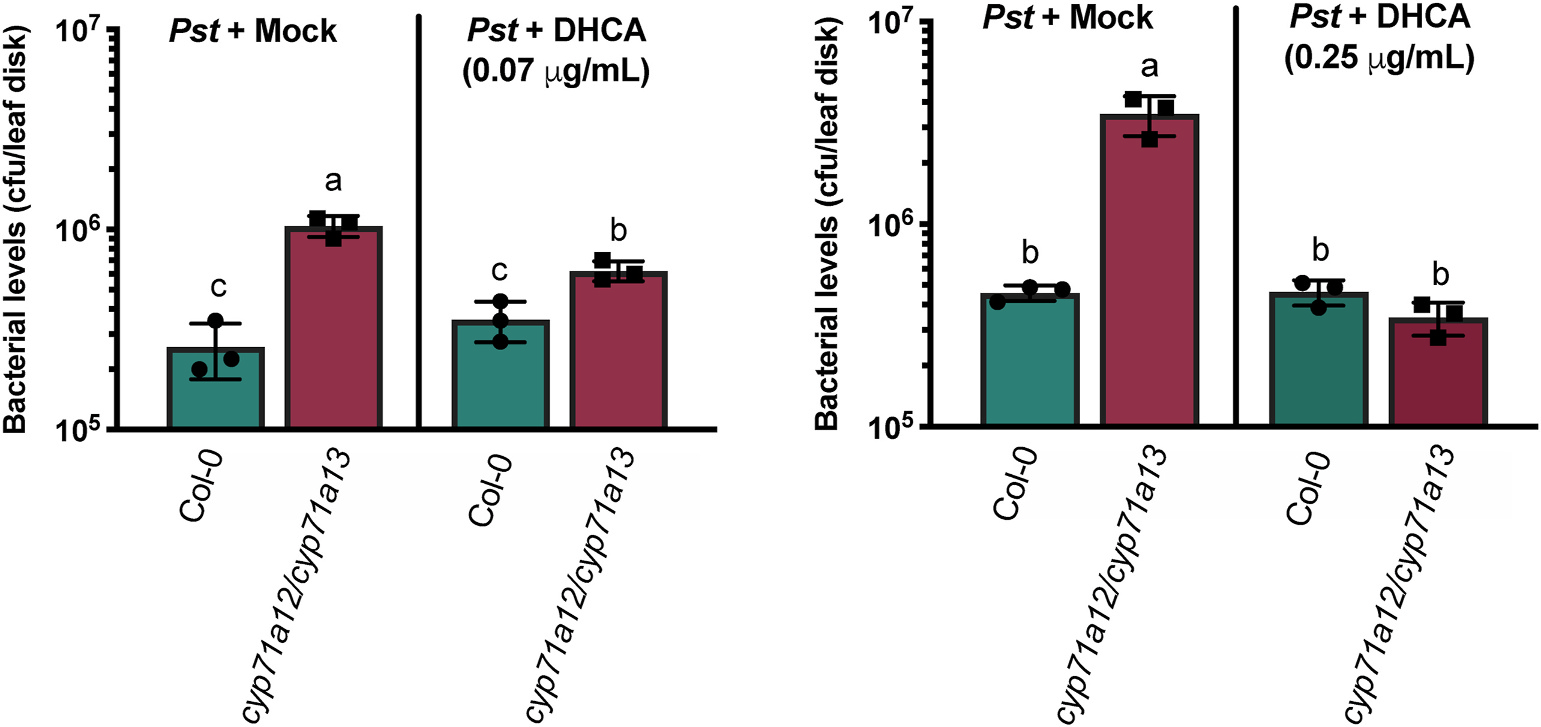
Exogenous infiltration of dihydroindole acid (DHCA) and bacterial quantification of P. syringae in 7-week-old Col-0 and cyp71a12/cyp71a13 rosette leaves.
CAS number: 6871-44-9
Echitamine is a natural alkaloid found in the bark of the Alstonia scholaris tree, also known as the devil's tree. It's known for its potential anti-cancer, anti-inflammatory, and antimicrobial properties. Research suggests it can inhibit cancer cell growth by inducing cell death (apoptosis), interfering with cell division, and disrupting mitochondrial function.
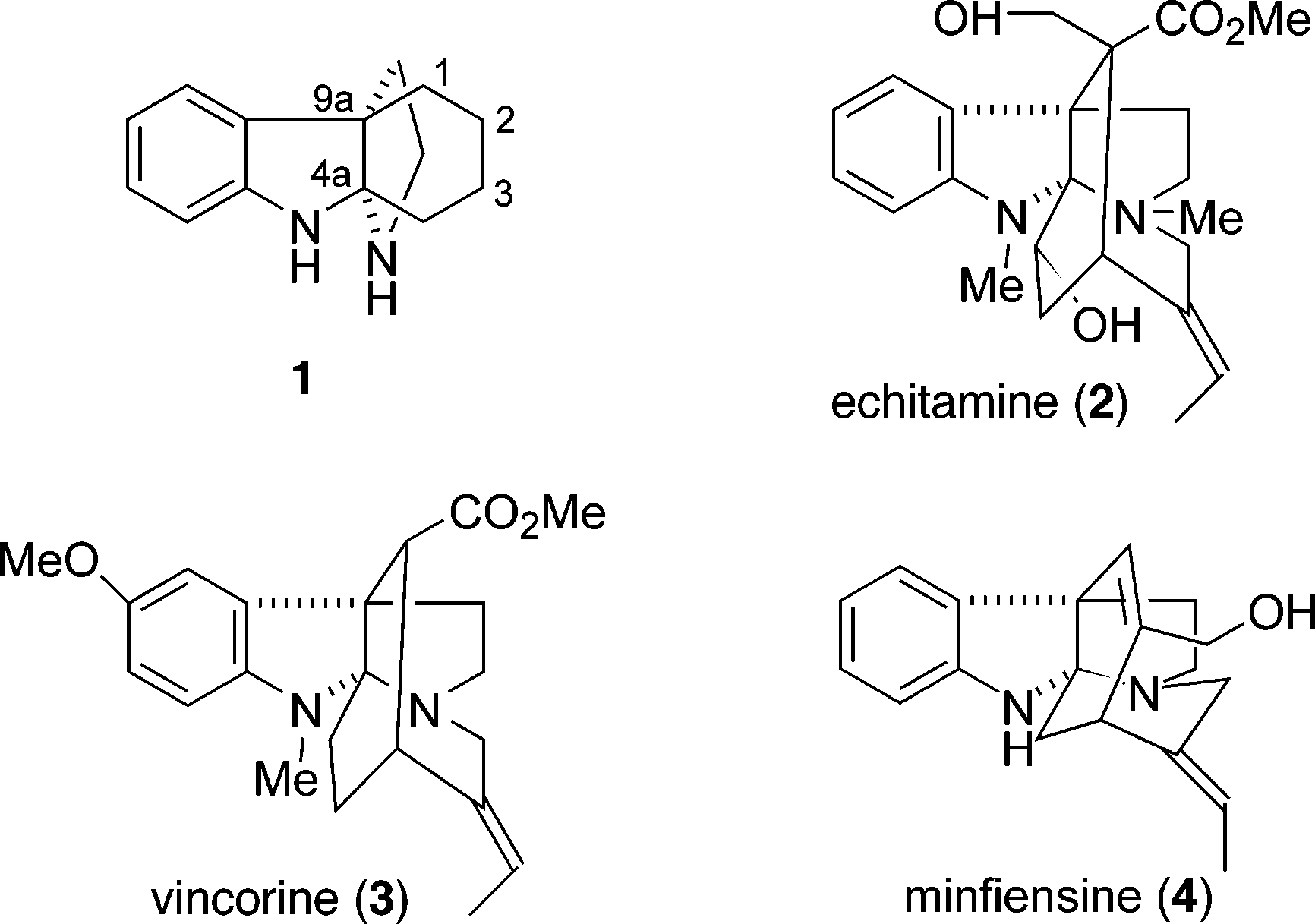
Representative alkaloids containing a 1,2,3,4-tetrahydro-9a,4a-(iminoethano)-9H-carbazole ring system: Echitamine, Vincorine, Minfiensine.
CAS number: 6893-02-3
Liothyronine is a thyroidal hormone T3 which is normally produced by the thyroid gland in a ratio 4:1 when compared with T4: T3. Liothyronine is the active form of thyroxine which is composed in a basic chemical structure by a tyrosine with bound iodine. The exogenous liothyronine product was developed by King Pharmaceuticals and FDA approved in 1956.
Selected haloarenes: Pestalachloride D, 2,4,6-Tribromo-m-cresol, Eosine Y, Liothyronine, Chlorpyrifos, mC21-A.
CAS number: 6894-38-8
Jasmonic acid is an oxo monocarboxylic acid that is (3-oxocyclopentyl)acetic acid substituted by a (2Z)-pent-2-en-1-yl group at position 2 of the cyclopentane ring. It has a role as a plant metabolite and a member of jasmonates. It is a conjugate acid of a jasmonate(1-). It is an enantiomer of a (+)-jasmonic acid.
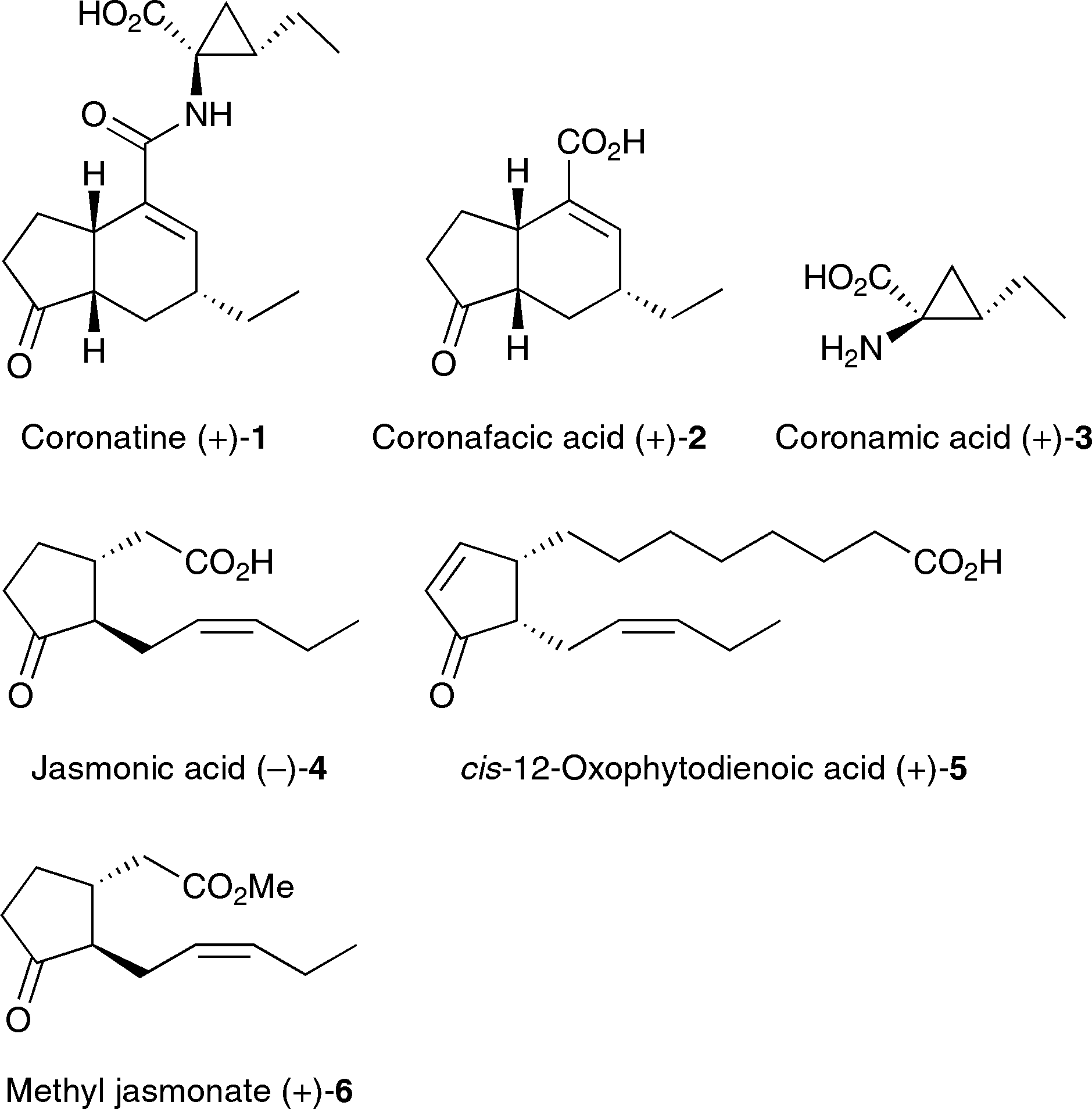
Structures of coronatine (+)-1, jasmonic acid (-)-4, and their derivatives.
CAS number: 69-72-7
Salicylic acid is a beta hydroxy acid (BHA) primarily known for its use in skincare, particularly for treating acne and other skin conditions.

Effects of dihydrocarbamazepine (DHCA) and salicylic acid (SA) on biofilm formation of Pseudomonas syringae (Pst) in vitro
CAS number: 691-37-2
4-Methyl-1-pentene is a hydrocarbon. It appears as a colorless liquid.
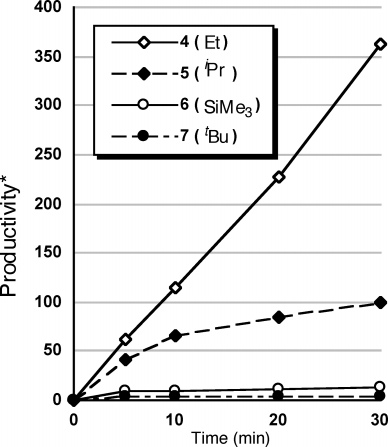
Production of 4-methyl-1-pentene catalyzed by complexes 4, 5, 6, and 7.
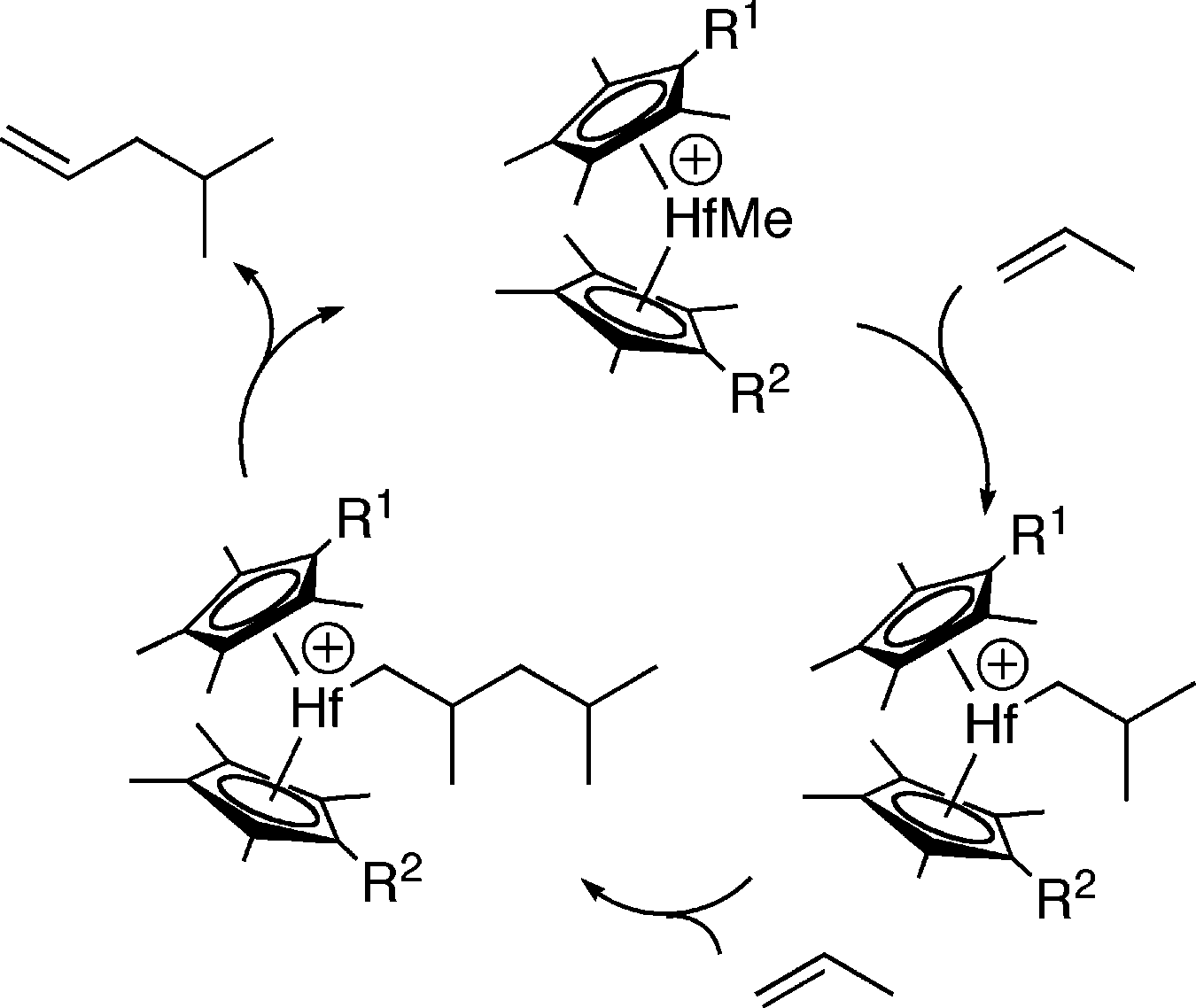
Idealized Catalytic Cycle for 4-Methyl-1-pentene Synthesis