Chemicals list & Research Gallery
CAS number: 57-47-6
A cholinesterase inhibitor that is rapidly absorbed through membranes. It can be applied topically to the conjunctiva. It also can cross the blood-brain barrier and is used when central nervous system effects are desired, as in the treatment of severe anticholinergic toxicity.
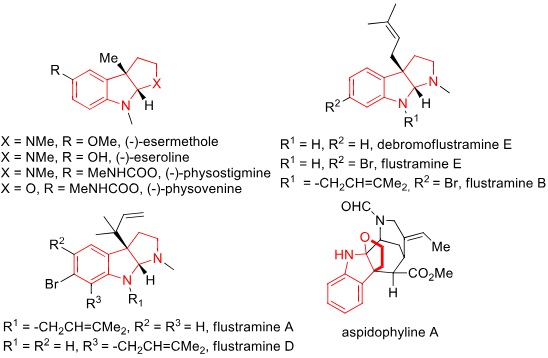
Indole Alkaloids Bearing FIH Skeletons: (-)-Esermethole, (-)-Eseroline, (-)-physostigmine, (-)-Physovenine, Flustramine E, Flustramine B, Flustramine A, Aspidophyline A.
CAS number: 57-48-7
D-fructopyranose is a fructopyranose having D-configuration. It has a role as a sweetening agent. It is a fructopyranose, a D-fructose and a cyclic hemiketal.
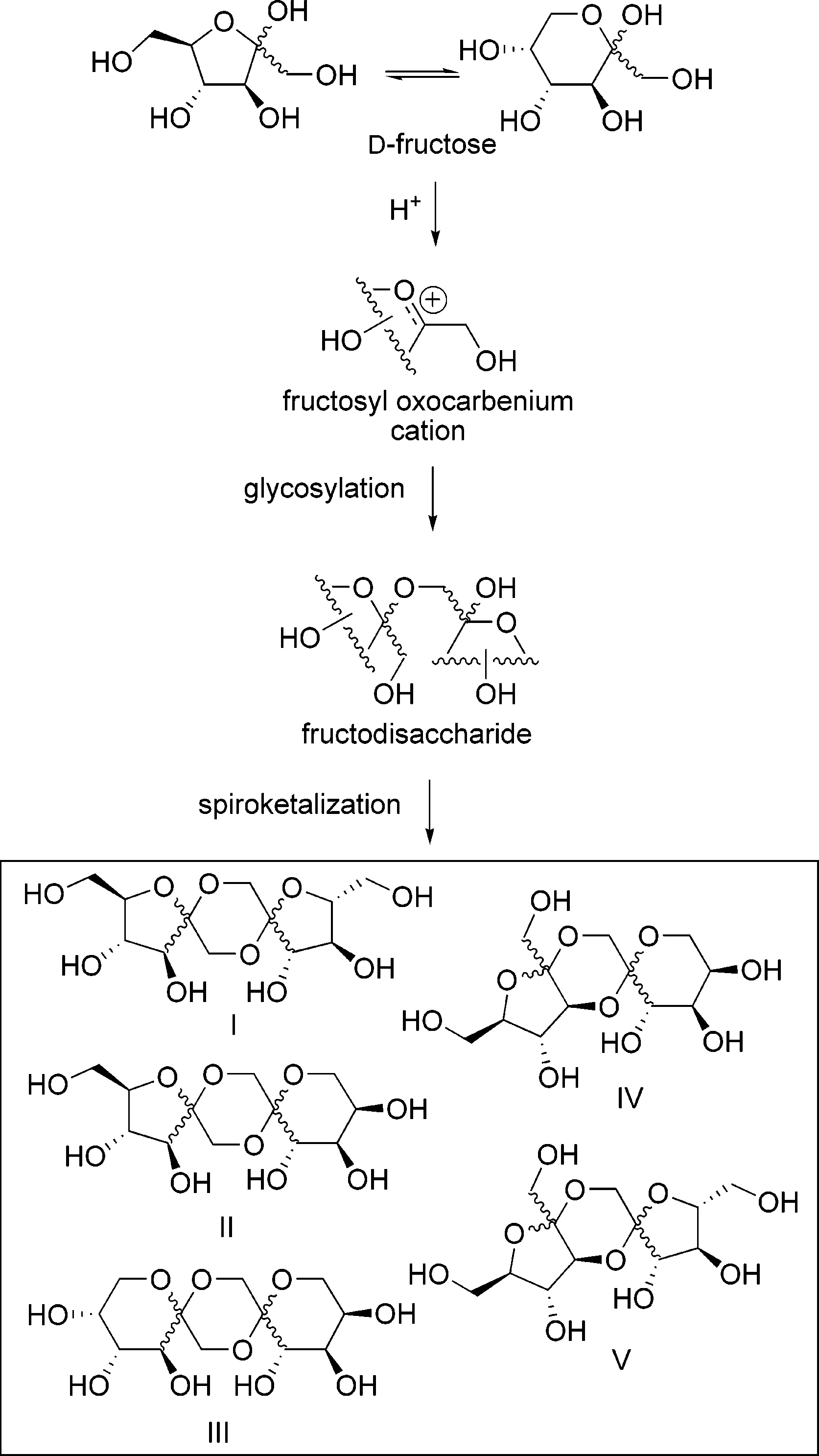
Spiroacetal Disaccharide Cores (Types I-V) Formed by Acid Treatment of D-Fructose
CAS number: 57-56-7
Semicarbazide is a monocarboxylic acid amide that is urea where one of the amino groups has been replaced with hydrazine. It is a monocarboxylic acid amide, a one-carbon compound, a member of ureas and a carbohydrazide.
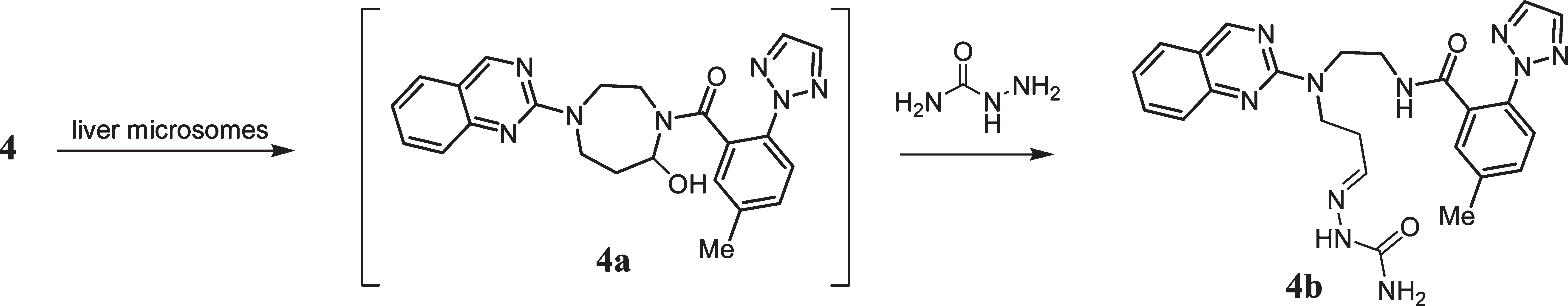
Proposed Mechanism of Semicarbazide Trapping When 4 Is Incubated in Liver Microsomes Fortified with Semicarbazide
CAS number: 57-88-5
Cholesterol is a cholestanoid consisting of cholestane having a double bond at the 5,6-position as well as a 3beta-hydroxy group. It has a role as a human metabolite, a mouse metabolite, a Daphnia galeata metabolite and an algal metabolite.
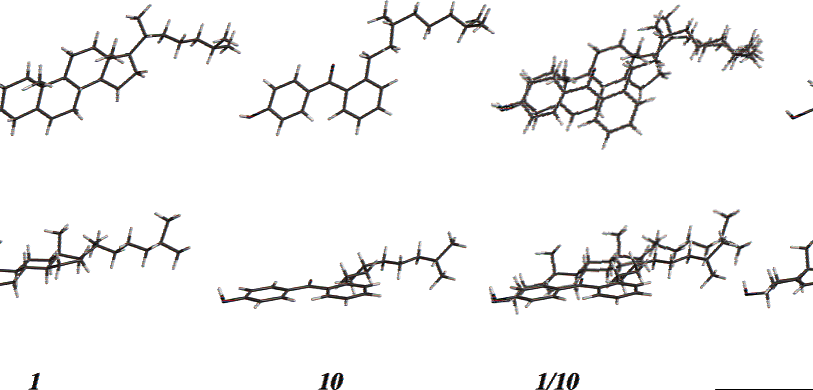
Spartan molecular modeling structures of cholesterol (1), analogue 10, and 1 superimposed on 10 in top and side views. The structure of 1 was energy minimized and that of 10 was conformationally manipulated for most effective overlap using Dreiding models as a guide.
CAS number: 57-92-1
Streptomycin, an antibiotic derived from Streptomyces griseus, was the first aminoglycoside to be discovered and used in practice in the 1940s. Selman Waksman and eventually Albert Schatz were recognized with the Nobel Prize in Medicine for their discovery of streptomycin and its antibacterial activity.
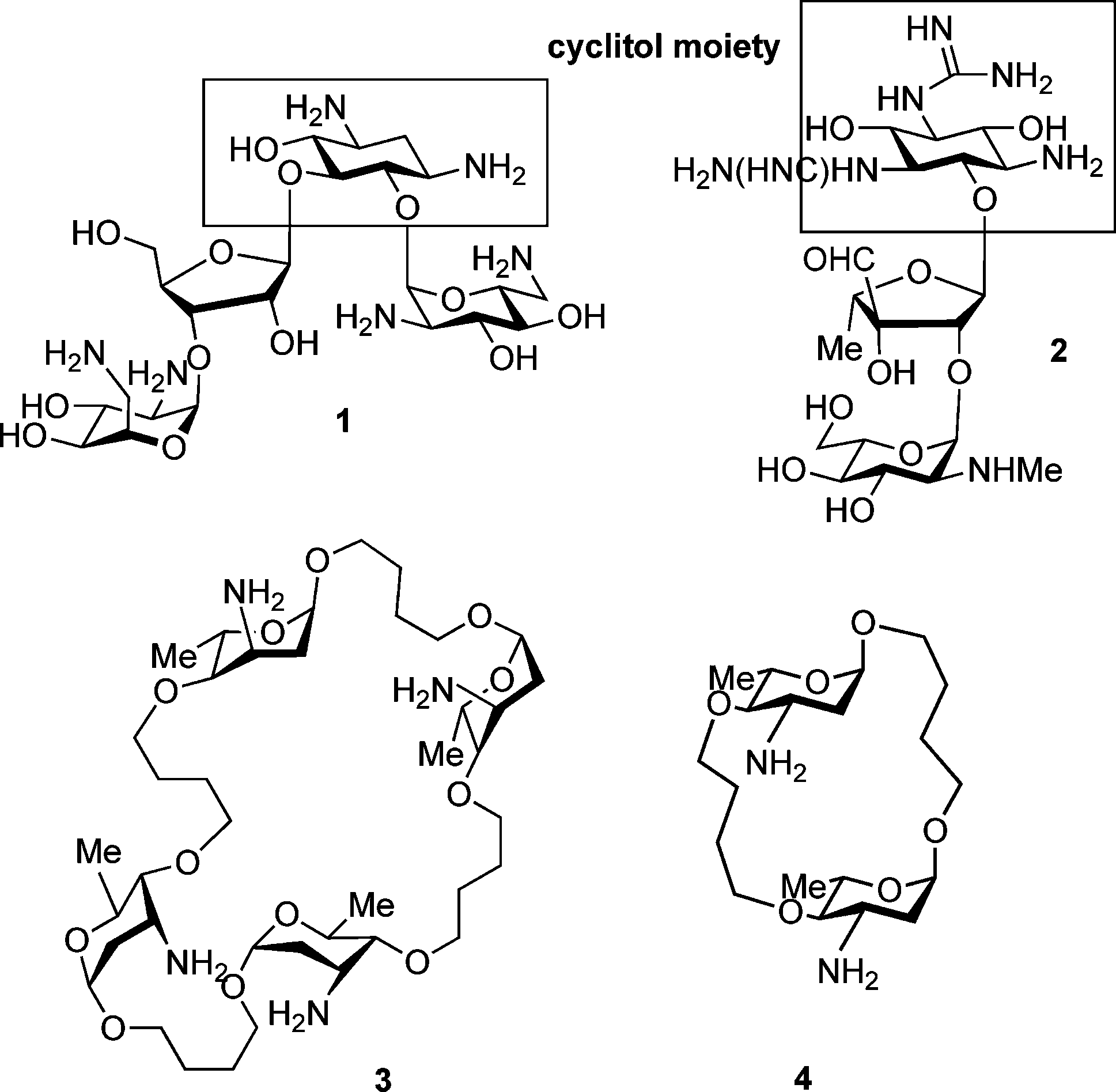
Structures of neomycin (1) and streptomycin (2), and macrocyclic neoaminoglycosides 3 and 4.
CAS number: 57072-36-3
Queuosine is a nucleoside found in tRNA that has an additional cyclopentenyl ring added via an NH group to the methyl group of 7-methyl-7-deazaguanosine. The cyclopentenyl ring may carry other substituents.
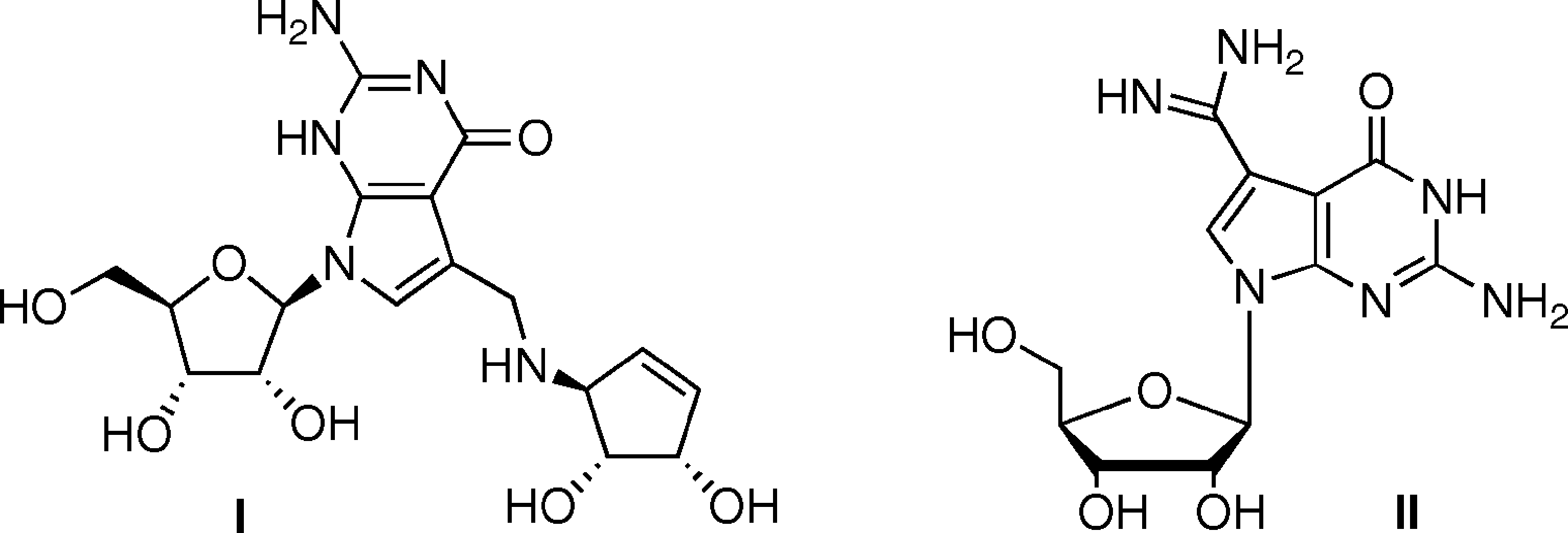
Queuosine (I) and Archaeosine (II).
CAS number: 571190-30-2
Palbociclib is a piperazine pyridopyrimidine that acts in the cell cycle machinery. It is a second generation cyclin-dependent kinase inhibitor selected from a group of pyridopyrimidine compounds due to its favorable physical and pharmaceutical properties. Palbociclib was developed by Pfizer Inc after the discovery that identified the cyclin-dependent kinases as key regulators of cell growth. It was originally FDA approved on March 2015 for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer and its indications were updated in April 2019 to include male patients based on findings from postmarketing reports and electronic health records demonstrating safety and clinical efficacy.
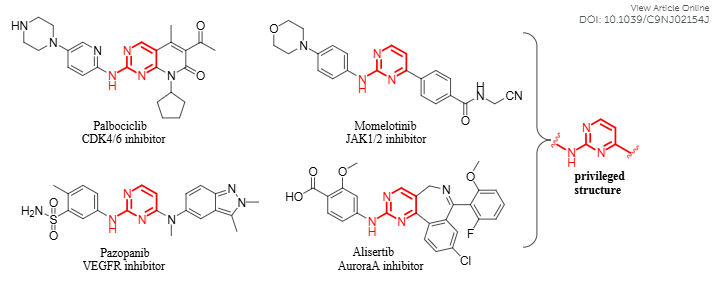
Structures of representative kinase inhibitors containing aminopyrimidine nuclei: Palbociclib, Momelotinib, Pazopanib, Alisertib
CAS number: 5720-05-8
p-Tolylboronic acid is used in the development of advanced materials, including polymers and nanomaterials, enhancing properties such as conductivity and strength, which are vital in electronics and coatings.
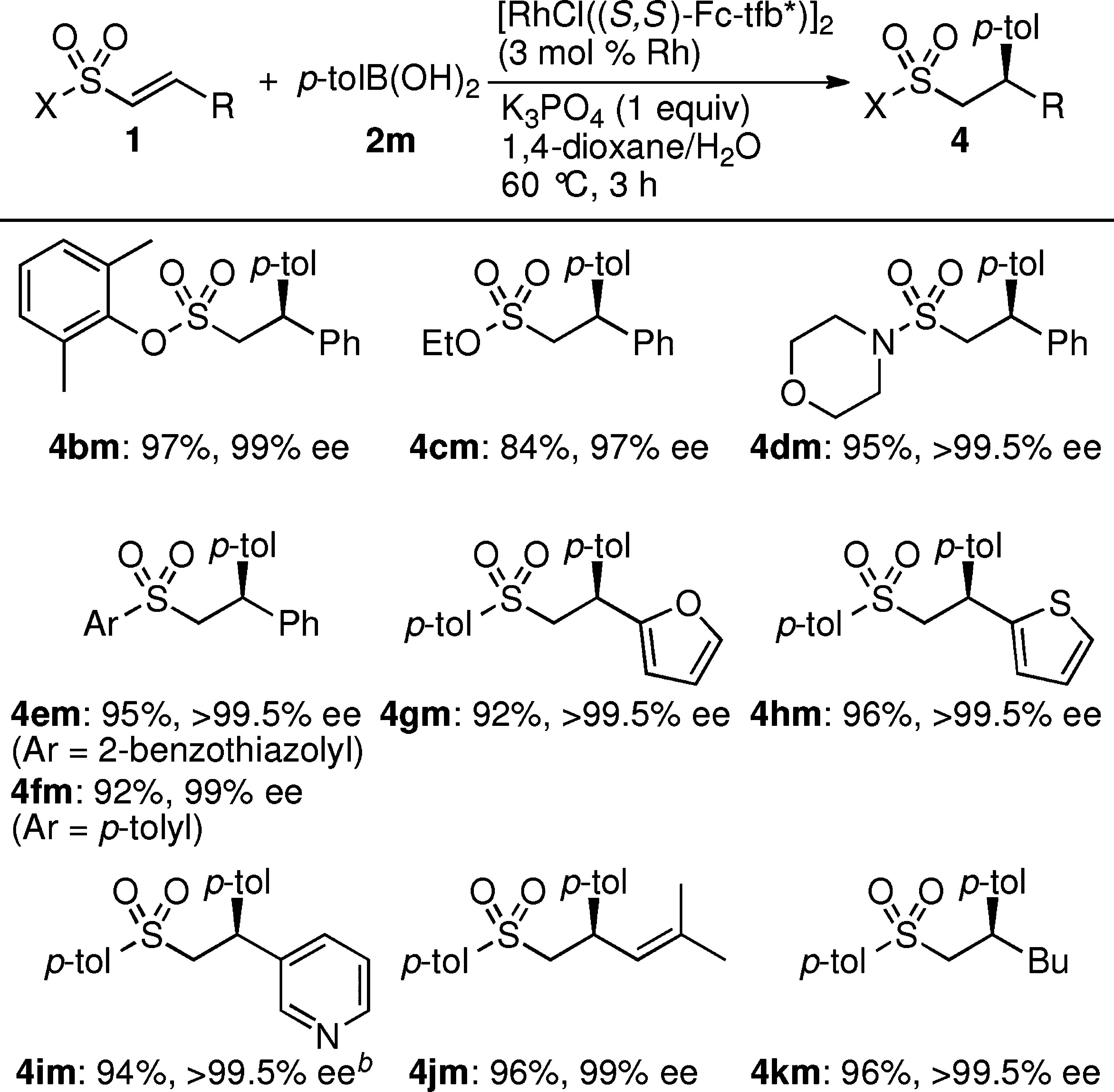
Rhodium-Catalyzed Asymmetric Addition of p-Tolylboronic Acid (2m) to 1α
CAS number: 57348-12-6
Zirconium molybdate is a chemical compound formed by the reaction of zirconium and molybdate ions, often found as a precipitate in nuclear fuel reprocessing solutions. It has the formula ZrMo2O7(OH)2(H2O)2 and can precipitate out of nitric acid solutions during the dissolution of spent nuclear fuel. It's also known to be a component of catalysts used in various chemical reactions.
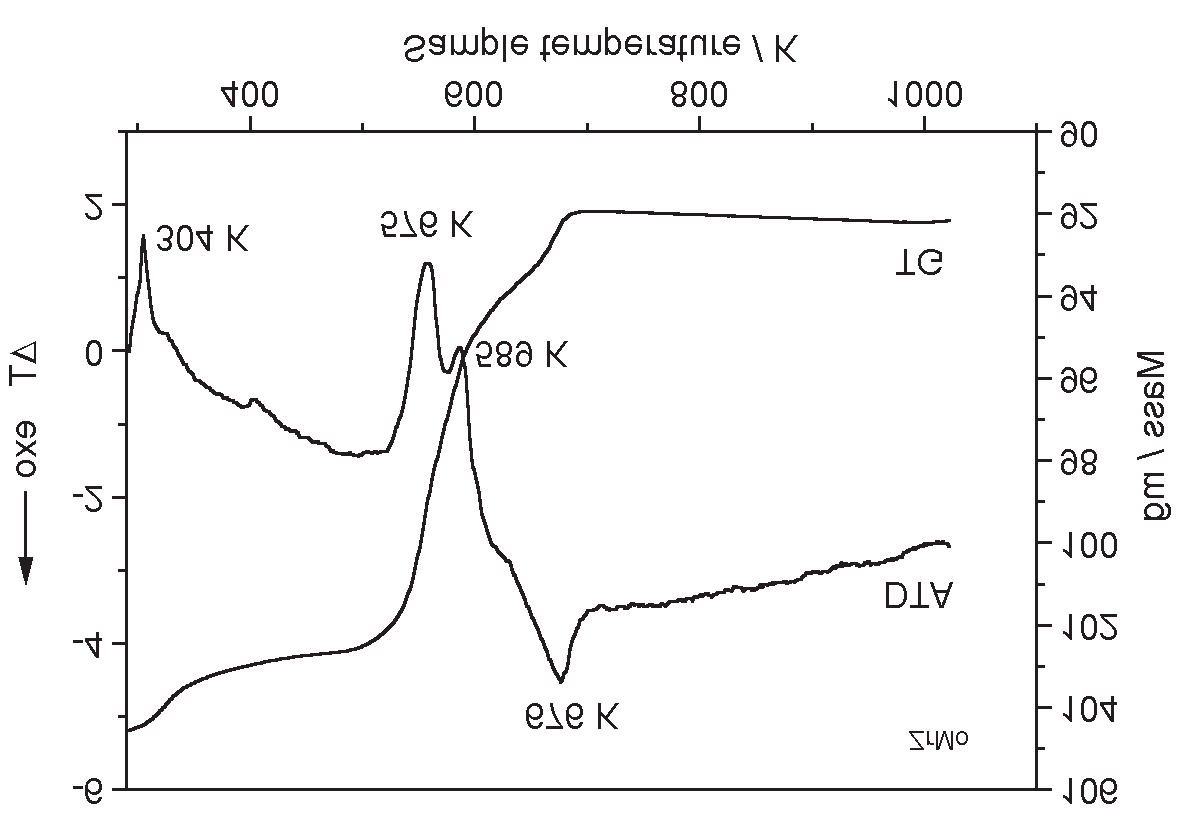
TG, DTA curves of zirconium molybdate
CAS number: 577-85-5
Flavonols are phytoconstituents of biological and medicinal importance.
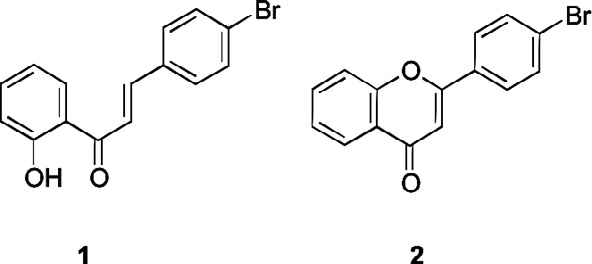
Flavonoid derivatives used in this study
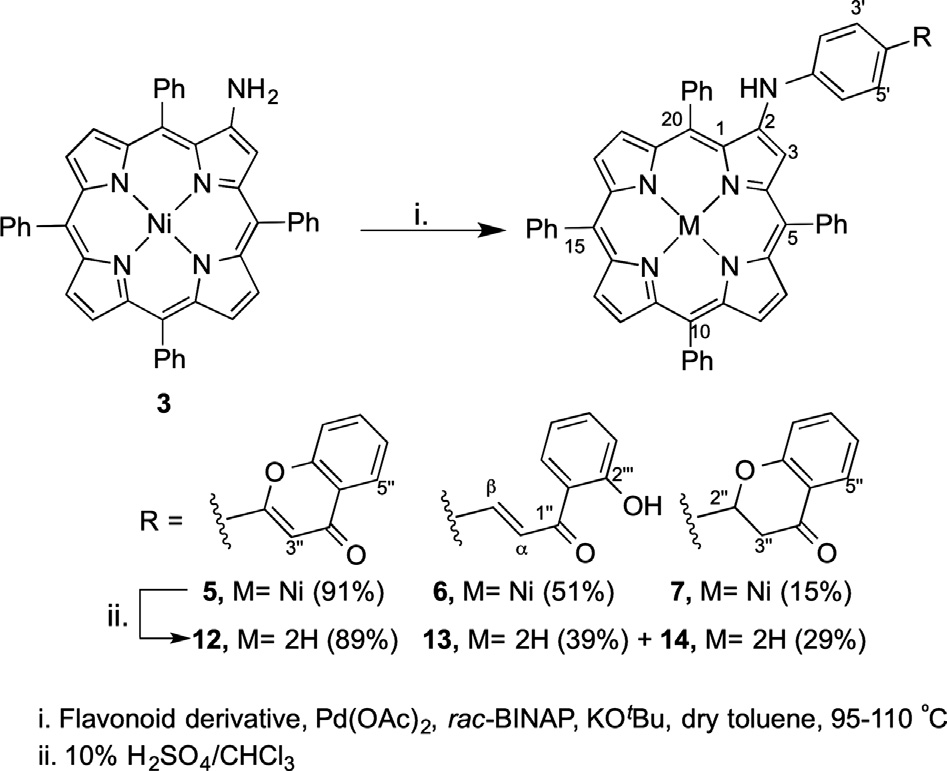
Synthetic strategy to prepare flavonoid–porphyrin conjugates at β-position.
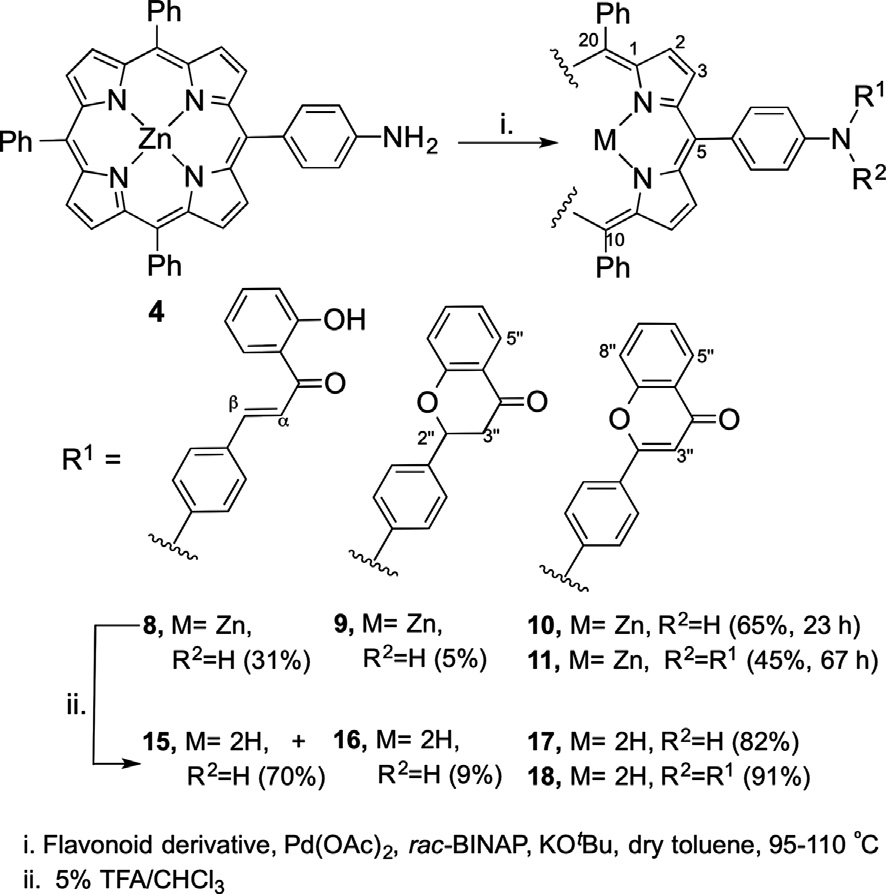
Synthetic strategy to prepare flavonoid–porphyrin conjugates at meso-position.
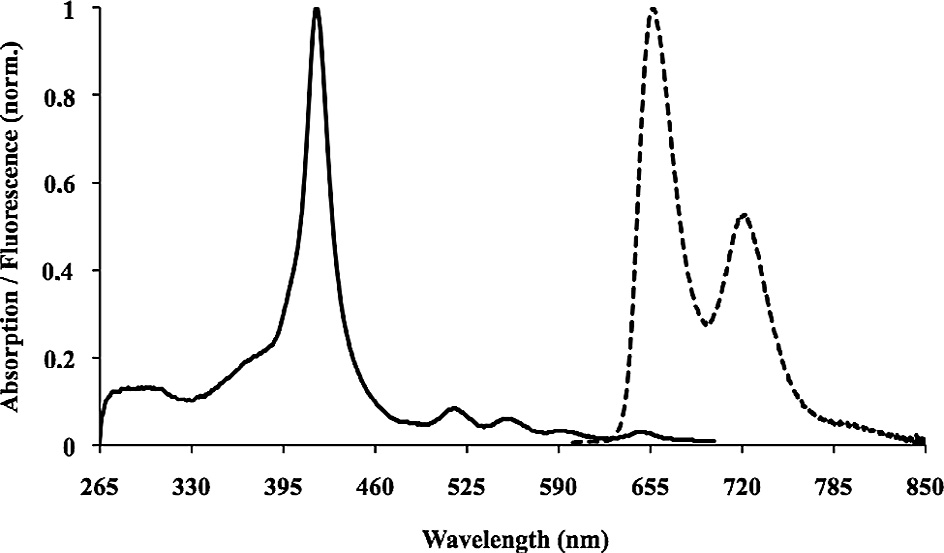
Representative normalized absorption (solid) and fluorescence spectra (dotted) (kexc = 550 nm, OD = 0.02) of flavonoid–porphyrin conjugate 17 in DMF.
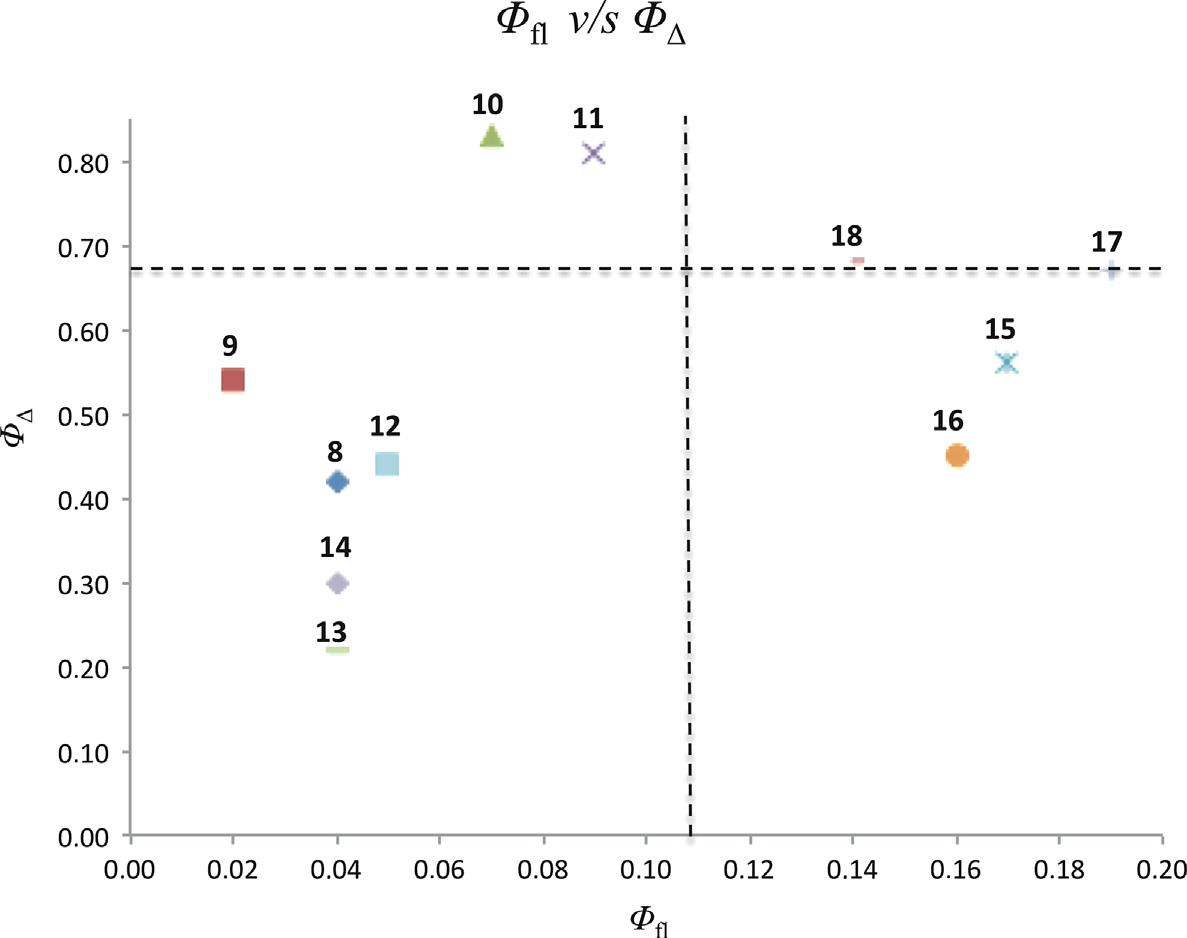
Plot of the singlet oxygen quantum yield (Ø△) of the flavonoid–porphyrin conjugates versus their fluorescence quantum yield (Øfl) in DMF. The interception of the dotted lines represents the value of the standard (TPP).
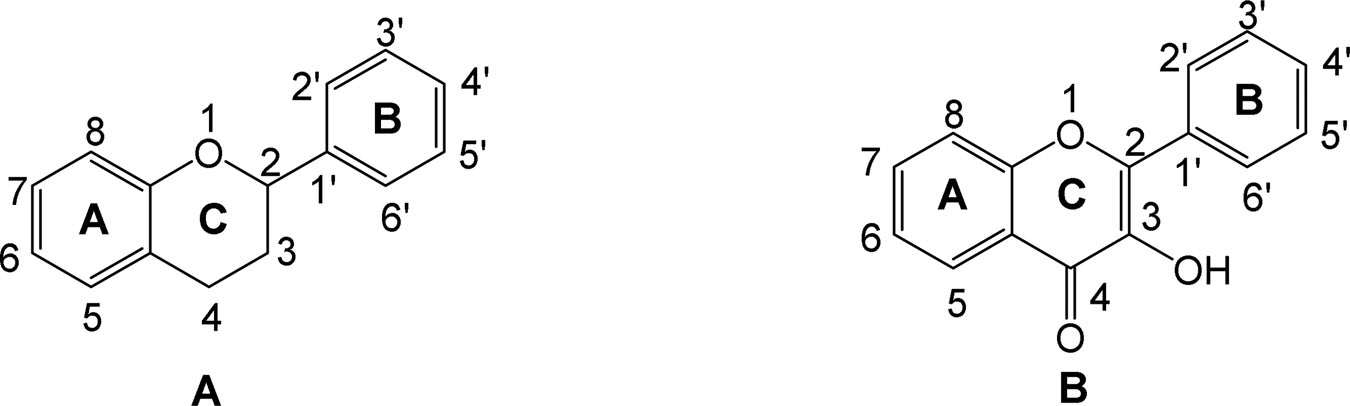
General structure of flavonoids (A) and 3-Hydroxyflavone (Flavonol) (B).
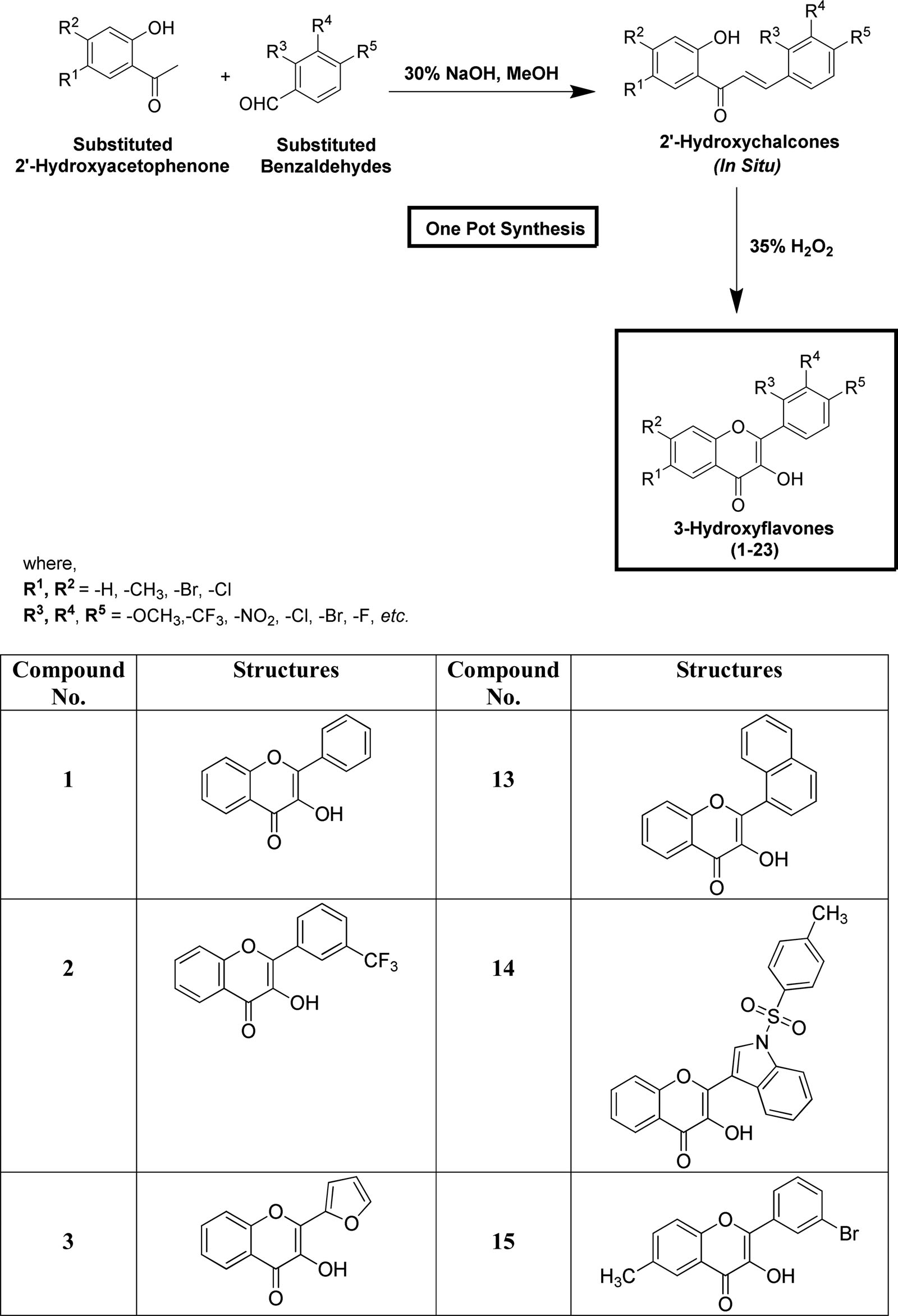
Synthesis of varyingly substituted 3-hydroxyflavone derivatives (1-23).