Chemicals list & Research Gallery
CAS number: 4942-61-4
(1S,2R,3S,4R)-cyclohex-5-ene-1,2,3,4-tetrol is a specific stereoisomer of cyclohex-5-ene-1,2,3,4-tetrol, also known as conduritol. It is a molecule with a cyclohexene ring and four hydroxyl (-OH) groups attached to carbons 1, 2, 3, and 4.
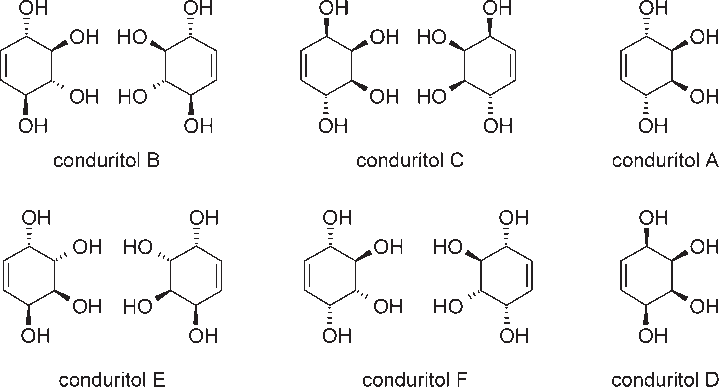
Structures of all conduritol isomers.
CAS number: 495-59-0
Deoxypeganine is a member of quinazolines.
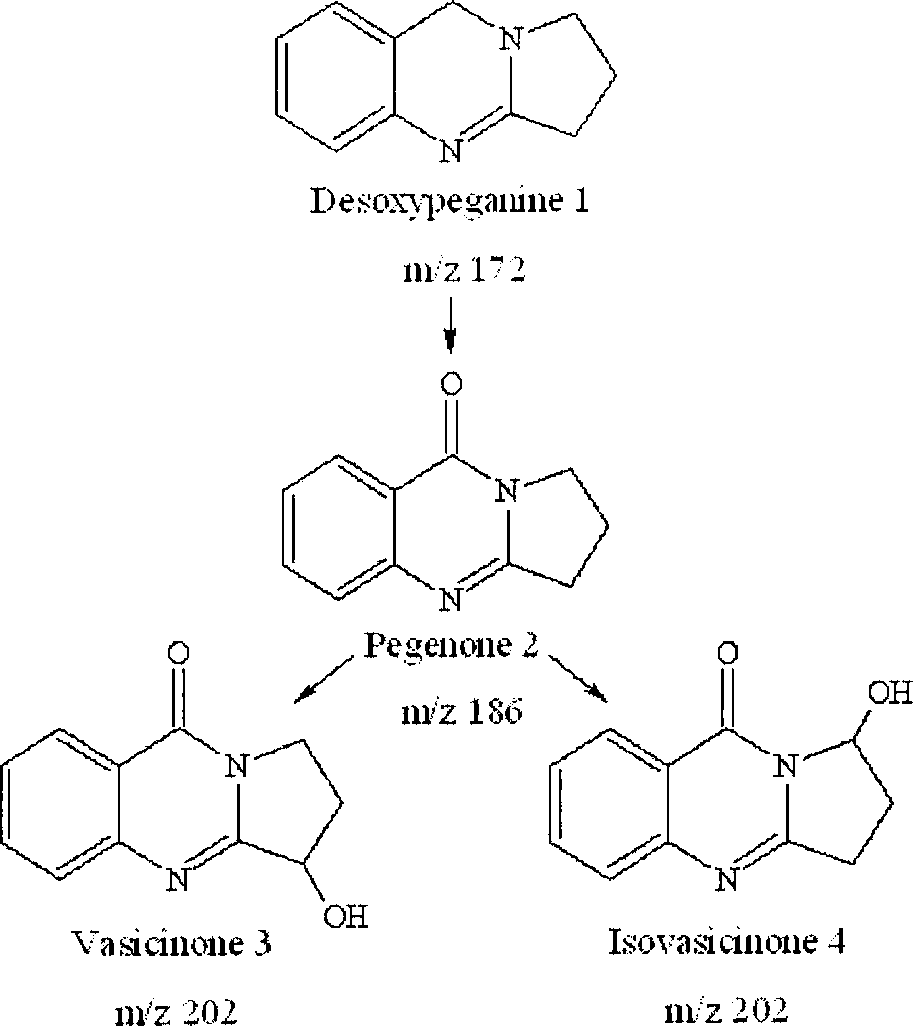
Proposed main metabolic pathway for Desoxypeganine 1 by microsomal enzymes.
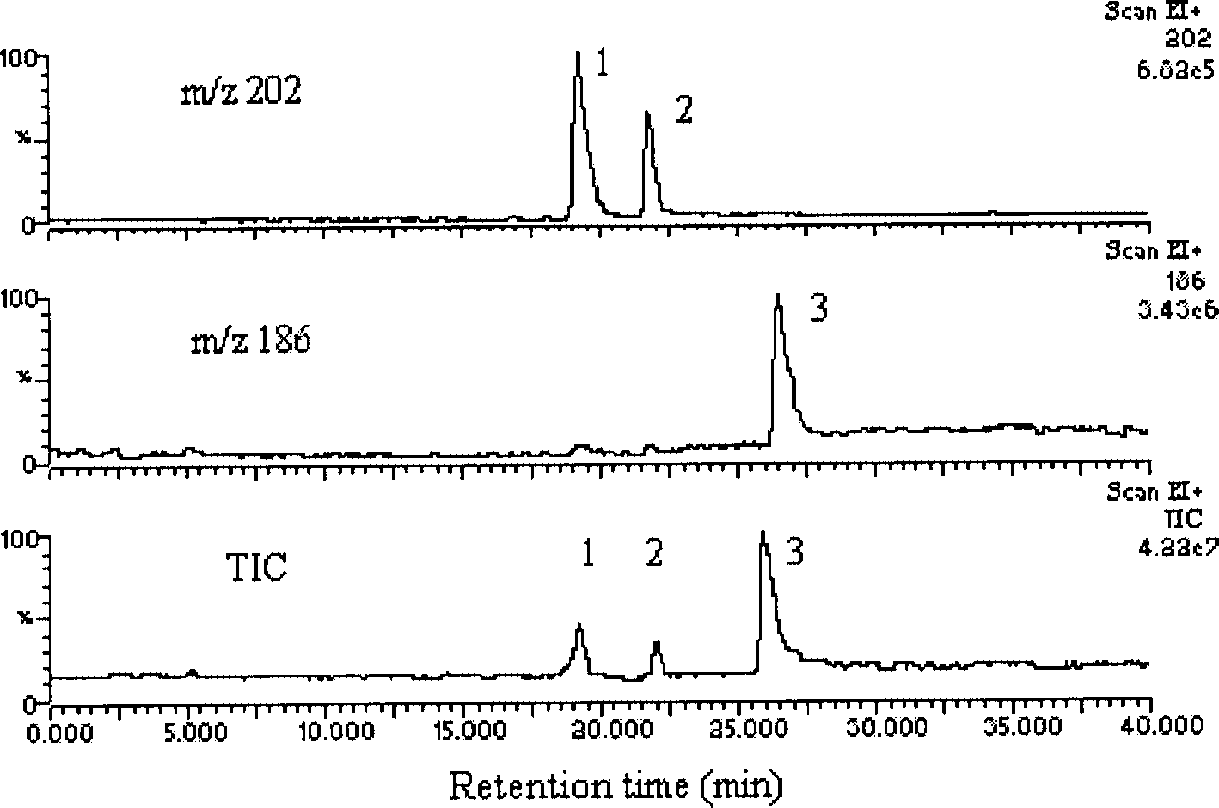
Total ion chromatogram and extracted mass chromatograms obtained from an incubation mixture with Desoxypeganine 1. Peak 1 Vasicinone 3, Peak 2 Isovasicinone 4, Peak 3 Pegenone 2.
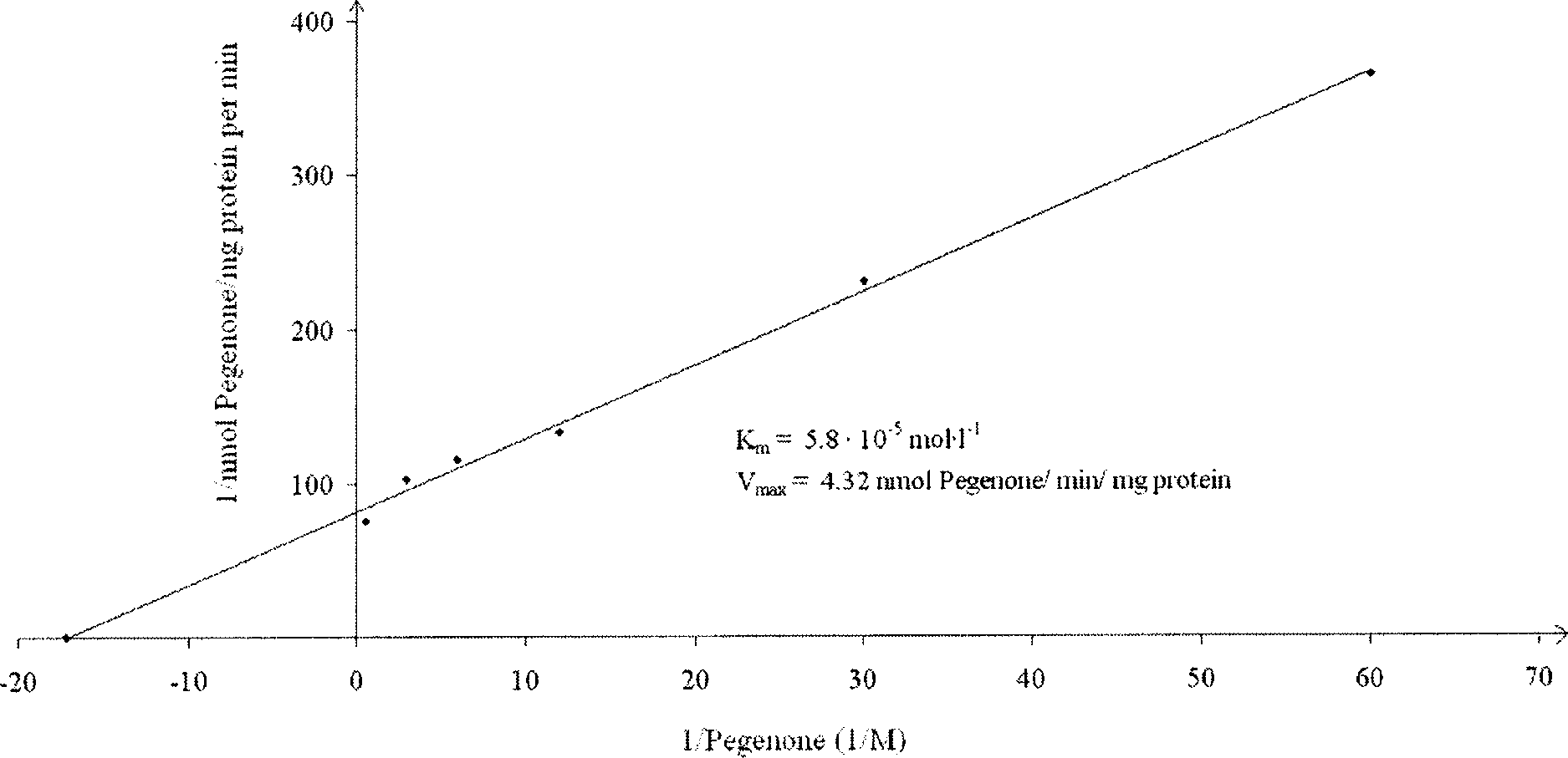
Lineweaver-Burk plot of C-oxidation of Desoxypeganine 1 measured by Pegenone 2 formation in the incubation mixtures containing the components described under Materials and methods. Mean values are presented from three determinations.
CAS number: 496-15-1
Indoline is a heterocyclic organic compound, which is a bicyclic structure formed by the fusion of a benzene ring and a five-membered nitrogen-containing ring (pyrrolidine ring).
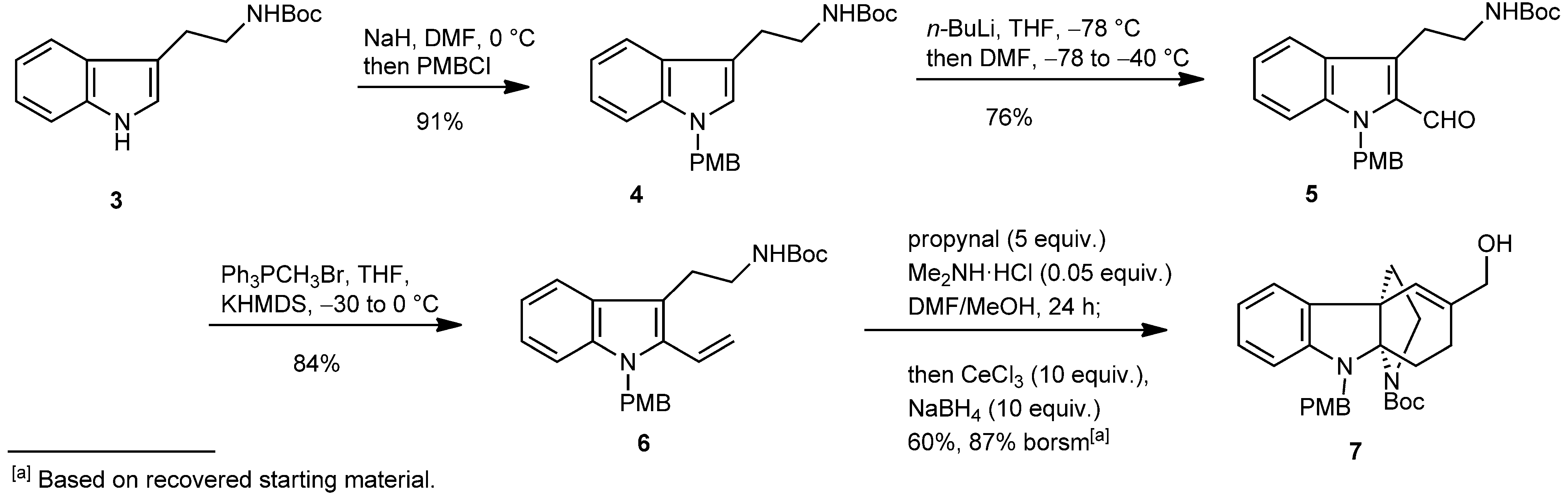
Synthesis of indoline 7.
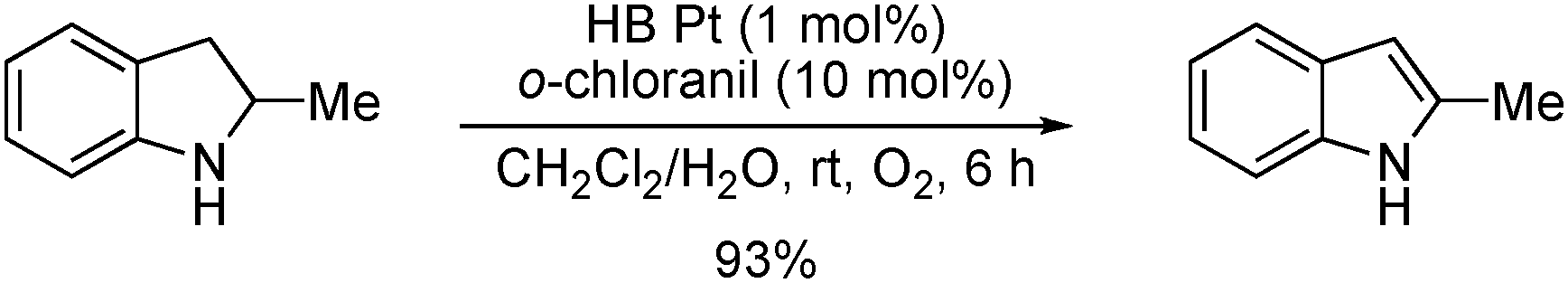
Aerobic oxidation of an indoline derivative.
CAS number: 497-30-3
Ergothioneine (ERG) is an unusual thio-histidine betaine amino acid that has potent antioxidant activities.
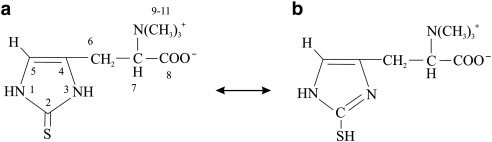
In solution, L-ergothioneine (EGT; 2-mercaptohistidine trimethylbetaine) exists as a tautomer between its thiol and thione forms.
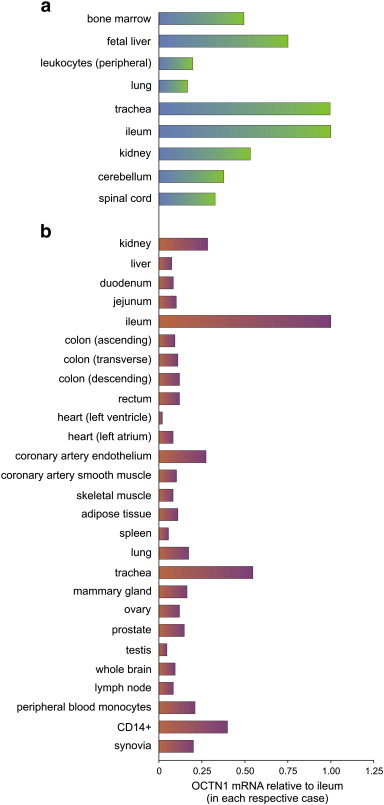
Tissue expression of the ERGOTHIONEINE transporter, OCTN1, analyzed by real-time PCR adapted from 2 independent sources (Grundemann et al. 2005; National Academy of Sciences and Taubert et al. 2009; BMJ Journals). Data are relative to mRNA level of the ileum for each set.
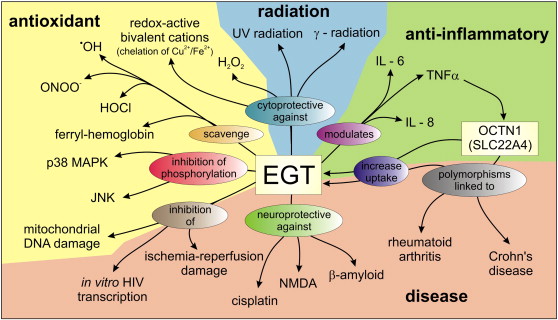
Possible roles and interactions of Ergothioneine in vivo. Functional roles as an antioxidant are highlighted in yellow, anti-inflammatory agent in green, protectant against radiation in blue and roles in disease in orange.
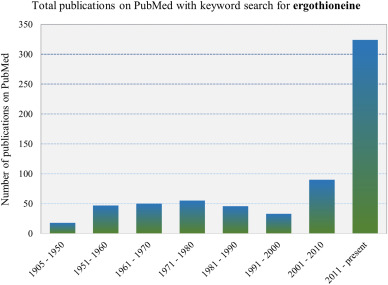
Since its first isolation in 1905, there was initially significant interest in Ergothioneine, however the interest waned until the discovery of its transporter (ETT/OCTN1) in 2005 and has since been the focus of intense research.
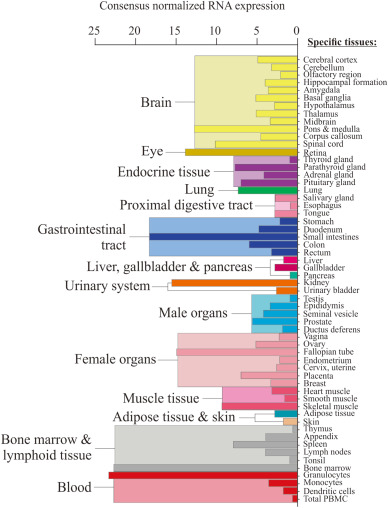
High expression of the transporter, and hence high levels of Ergothioneine, is observed in certain cells (e.g. blood cells, bone marrow, ocular tissues, brain etc.) that are likely predisposed to oxidative stress, although other tissues can accumulate high levels of ET with sustained administration.
CAS number: 498-66-8
Norbornene, also known as bicyclo[2.2.1]hept-2-ene, is a cyclic alkene with a dense three-dimensional structure: a cyclohexene ring with a bridging methylene in the para-position.
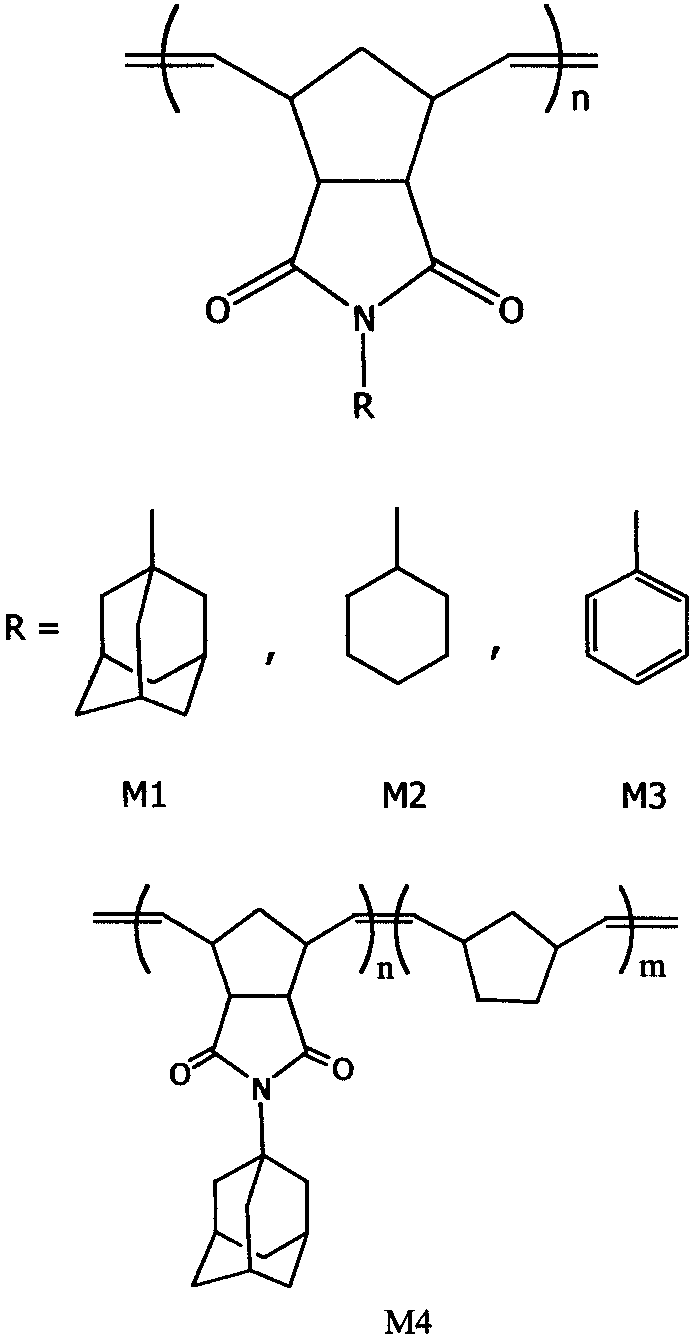
Schematic representation of the repeating units of poly(N-(1-adamantyl)-exo-norbornene-5,6-dicarboximide) (M1), poly(N-cyclohexyl-exo-norbornene-5,6-dicarboximide) (M2), poly-(N-phenyl-exo-norbornene-5,6-dicarboximide) (M3), and copolymers of N-(1-adamantyl)-exo-norbornene-5,6-dicarboximide with norbornene.
CAS number: 50-00-0
Formaldehyde was described in the year 1855 by the Russian scientist Alexander Michailowitsch Butlerow. At room temperature, formaldehyde is a colorless, flammable gas that has a distinct, pungent smell.
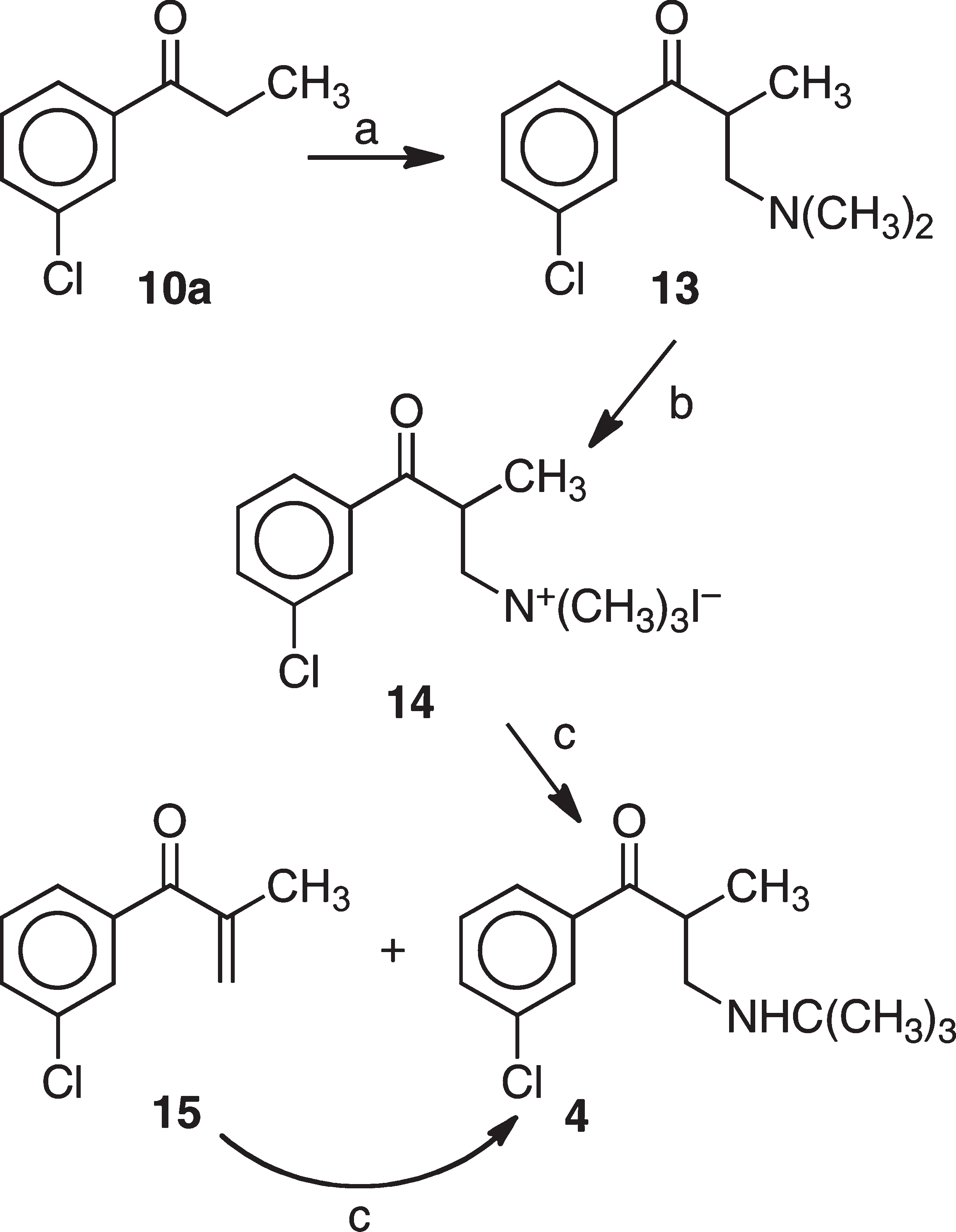
Scheme 3 shows the procedure used for the synthesis of 4. Subjection of 3'-chloropropiophenone (10a) to Mannich reaction conditions with aqueous formaldehyde and dimethylamine gave 13. The methiodide 14 was obtained by alkylation of 13 with iodomethane. Treatment of 14 with tert-butylamine gave first a mixture of 15 and the desired 4. Subjection of the mixture to excess tert-butylamine provided the desired target compound.
CAS number: 50-02-2
Dexamethasone, or MK-125, is a corticosteroid fluorinated at position 9 used to treat endocrine, rheumatic, collagen, dermatologic, allergic, ophthalmic, gastrointestinal, respiratory, hematologic, neoplastic, edematous, and other conditions. Developed in 1957, it is structurally similar to other corticosteroids like [hydrocortisone] and [prednisolone]. Dexamethasone was granted FDA approval on 30 October 1958. In a press release for the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial on 16 June 2020, dexamethasone was recommended for use in COVID-19 patients with severe respiratory symptoms. Dexamethasone reduced deaths by approximately one third in patients requiring ventilation and by one fifth in those requiring oxygen.
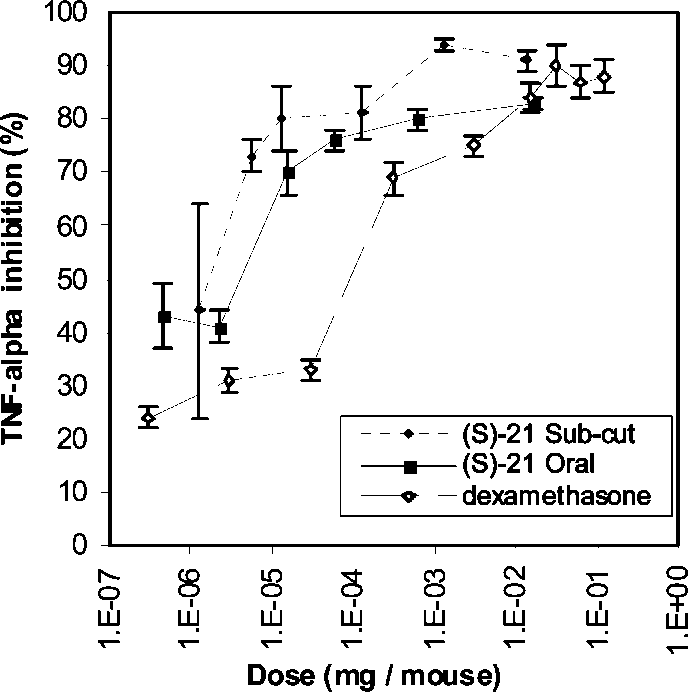
Inhibition of LPS induced TNF-R in vivo by (S)-21 (subcutaneous and oral dose) and dexamethasone (subcutaneous dose).
CAS number: 50-06-6
Phenobarbital is a member of the barbiturate drug class that holds versatile therapeutic applications. This drug is effective in anti-seizure management, treatment for status epilepticus, and insomnia; it also plays a pivotal role in addressing benzodiazepine and alcohol withdrawal. Phenobarbital (phenobarbitone) was first used as an antiepileptic drug 100 years ago, in 1912.
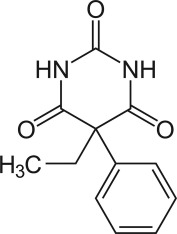
Structure formula of phenobarbital or 5-Ethyl-5-phenylbarbituric acid
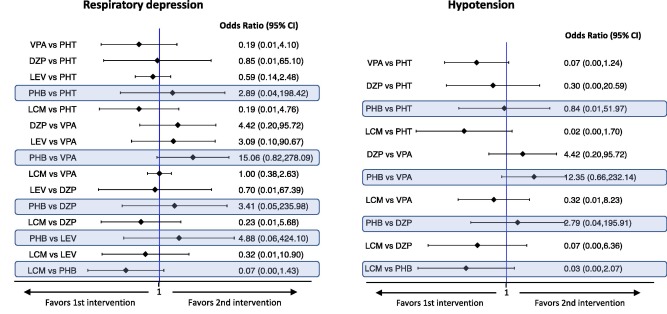
Interval plots for the safety outcomes of five studies in benzodiazepine-resistant Status epilepticus. Highlighted in blue are the comparisons including phenobarbital (From: Brigo et al. Epilepsy and Behavior 2019).
CAS number: 50-07-7
Mitomycin C is a mitomycin. It has a role as an antineoplastic agent and an alkylating agent. It is a conjugate acid of a mitomycin C(1-).
![Selected examples of natural products that contain the pyrrolo[1,2-a]-indole framework.](http://www.wlxkc.cn/picture/5235593_01.png)
Selected examples of natural products that contain the pyrrolo[1,2-a]-indole framework.
CAS number: 50-28-2
Estradiol is a naturally occurring hormone circulating endogenously in females. It is commercially available in several hormone therapy products for managing conditions associated with reduced estrogen, such as vulvovaginal atrophy and hot flashes. Some available forms of estradiol include oral tablets, injections, vaginal rings, transdermal patches, sprays, gels, and creams.
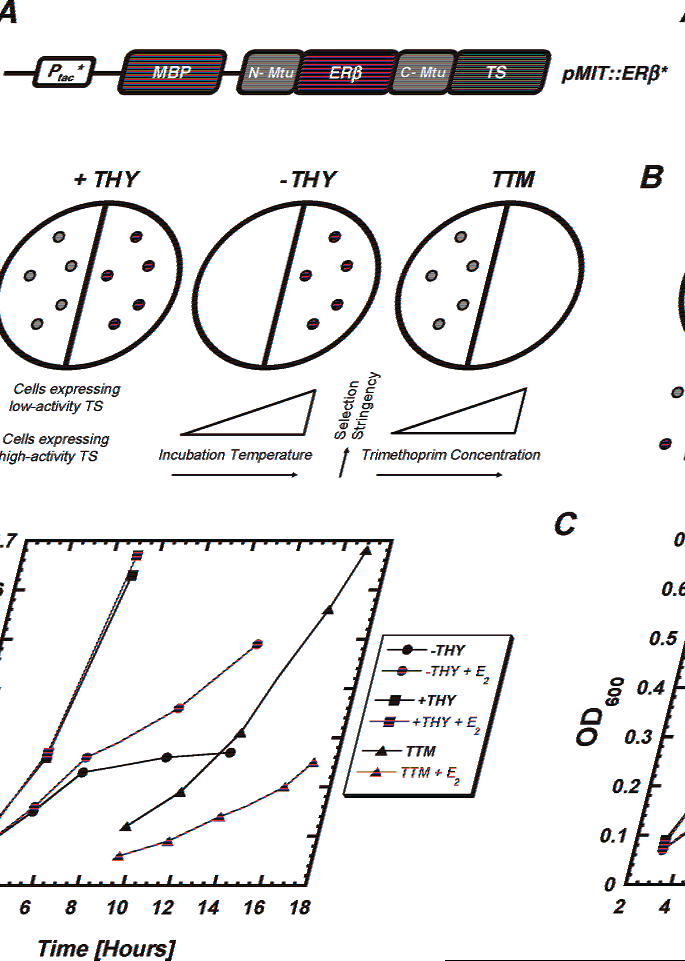
Design and associated growth phenotypes of the ERβ-based estrogen-sensing system.