Formaldehyde
CAS number: 50-00-0
Formaldehyde was described in the year 1855 by the Russian scientist Alexander Michailowitsch Butlerow. At room temperature, formaldehyde is a colorless, flammable gas that has a distinct, pungent smell.
Related images
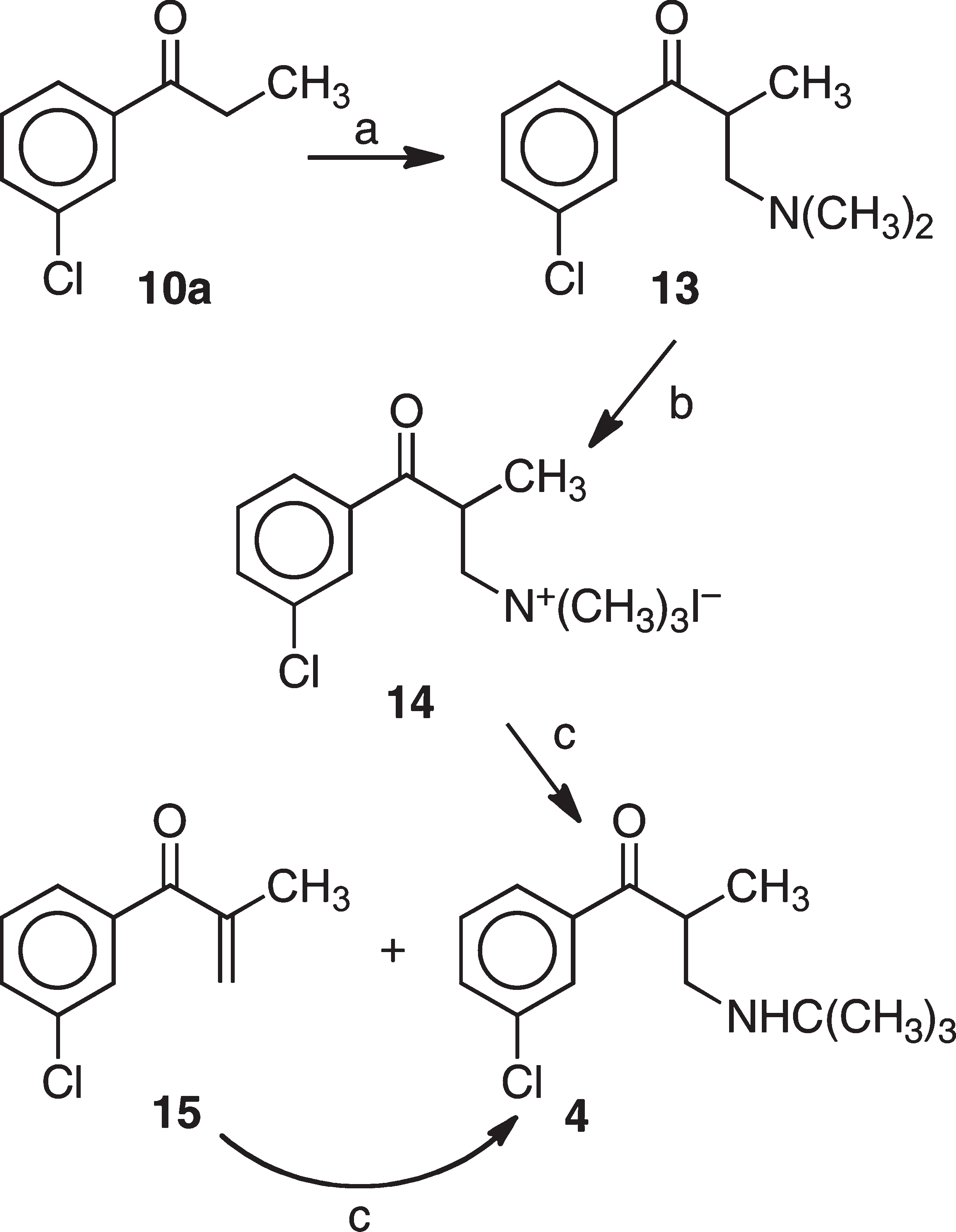
Scheme 3 shows the procedure used for the synthesis of 4. Subjection of 3'-chloropropiophenone (10a) to Mannich reaction conditions with aqueous formaldehyde and dimethylamine gave 13. The methiodide 14 was obtained by alkylation of 13 with iodomethane. Treatment of 14 with tert-butylamine gave first a mixture of 15 and the desired 4. Subjection of the mixture to excess tert-butylamine provided the desired target compound.
Related Questions and Answers
A: Formaldehyde is a significant environmental health risk because it is classified as a Group 1 human carcinogen by the IARC and is identified by the USEPA as one of the most critical hazardous air pollutants. Even low concentrations (e.g., 1 μg/m³) may elevate the risk of lung and nasopharyngeal cancers. Formaldehyde can also cause eye and respiratory tract irritation, nausea, fatigue, and in high exposures, serious conditions like emphysema and renal failure. Its health impact is exacerbated in warmer seasons due to increased photochemical formation from volatile organic compounds.
A: Formaldehyde impairs methanol assimilation in engineered E. coli strains by causing DNA-protein cross-links (DPCs), DNA strand breaks, and protein crosslinking. These damages inhibit cellular processes such as DNA replication and protein folding, thereby reducing cell growth and methanol utilization efficiency. The accumulation of intracellular formaldehyde, not methanol itself, was shown to be the major toxic factor limiting methanol assimilation. Experimental data indicated that 1.25 mM formaldehyde is lethal to E. coli, whereas the cells can tolerate methanol concentrations up to 2,400 mM, highlighting the critical need to alleviate formaldehyde toxicity for improved methanol assimilation.