Chemicals list & Research Gallery
CAS number: 4166-53-4
3-Methylglutaric anhydride is an organic compound classified as a cyclic anhydride, derived from 3-methylglutaric acid. It appears as a colorless to pale yellow solid and is moderately reactive due to the strained anhydride ring, making it useful as an intermediate in organic synthesis, particularly in the preparation of polymers, pharmaceuticals, and agrochemicals.
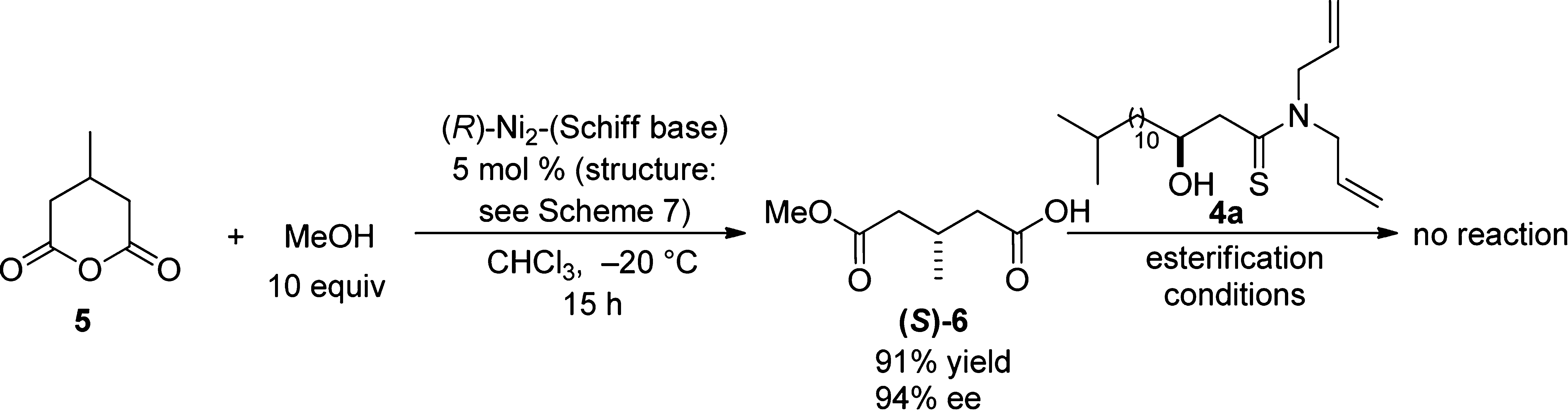
Desymmetrization of 3-Methylglutaric Anhydride with Methanol Followed by Esterification with Thioamide-Aldol Product 4a
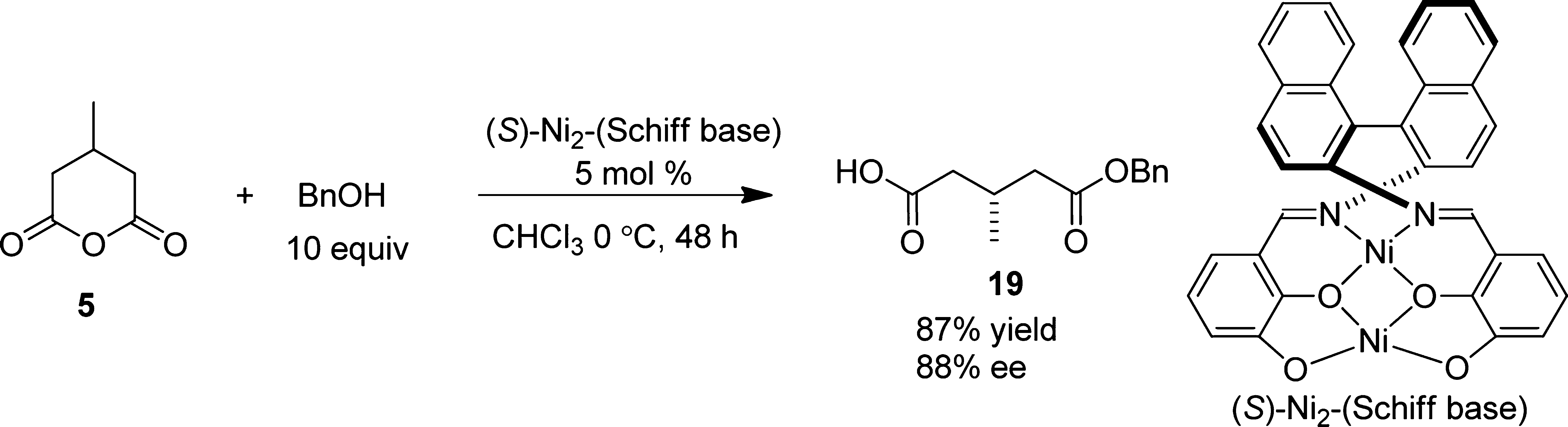
Desymmetrization of 3-Methylglutaric Anhydride 5 with Benzyl Alcohol
CAS number: 41661-47-6
4-Piperidone is a derivative of piperidine and is used as an intermediate in the production of various chemicals and pharmaceutical drugs.
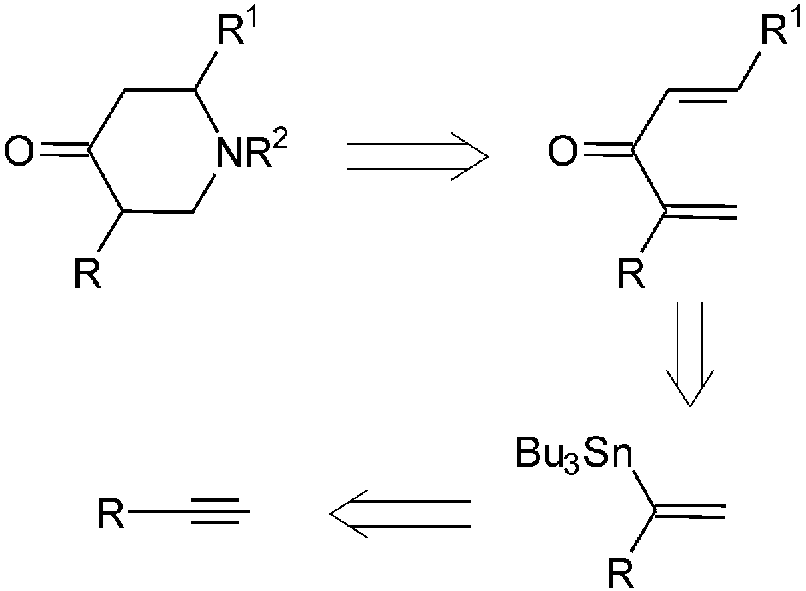
Retrosynthetic analysis of substituted 4-piperidones
CAS number: 4172-91-2
N-Methyl-N'-phenylcarbodiimide is a carbodiimide, a class of compounds characterized by the functional group -N=C=N-. Specifically, it features a methyl group (-CH3) and a phenyl group (C6H5) attached to the carbodiimide group.
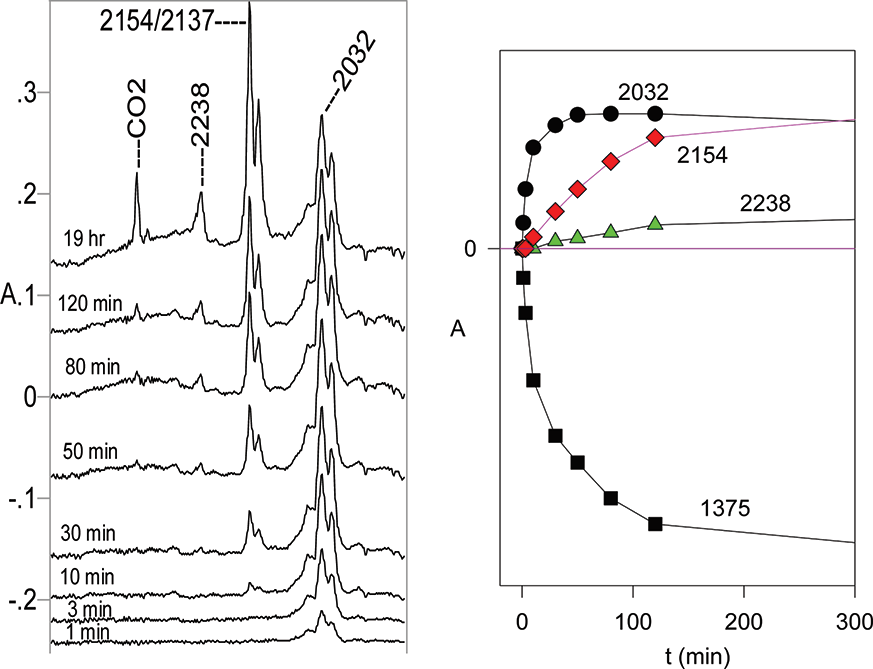
(Left) Partial IR difference spectra of tetrazole 16 at 12 K in Ar matrix at different photolysis times at 254 nm. The positive peaks are due to the photolysis products. The peaks at 2154 and 3137 cm−1 belong to the same compound, N-methyl-N′-phenylcarbodiimide 20. Abscissa 1900−2500 cm−1. (Right) Plots of IR absorbances at different wavenumbers versus photolysis time. The 1375 cm−1 band belongs to the starting material 16. Ordinates in arbitrary absorbance units.
CAS number: 4199-09-1
Levopropranolol, also known as (-)-propranolol or S-(-)-propranolol, is the active levorotatory isomer of the beta-blocker drug propranolol.
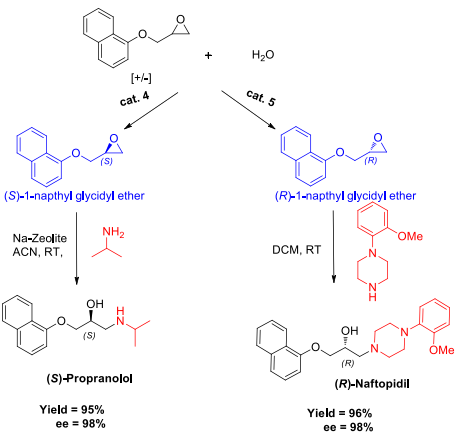
Synthesis of chiral drugs (S)-Propranolol and (R)-Naftopidil.
CAS number: 420-04-2
Cyanamide is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by an amino group. It has a role as an EC 1.2.1.3 [aldehyde dehydrogenase (NAD(+))] inhibitor. It is a nitrile and a one-carbon compound. It is a conjugate acid of a cyanamide(2-).
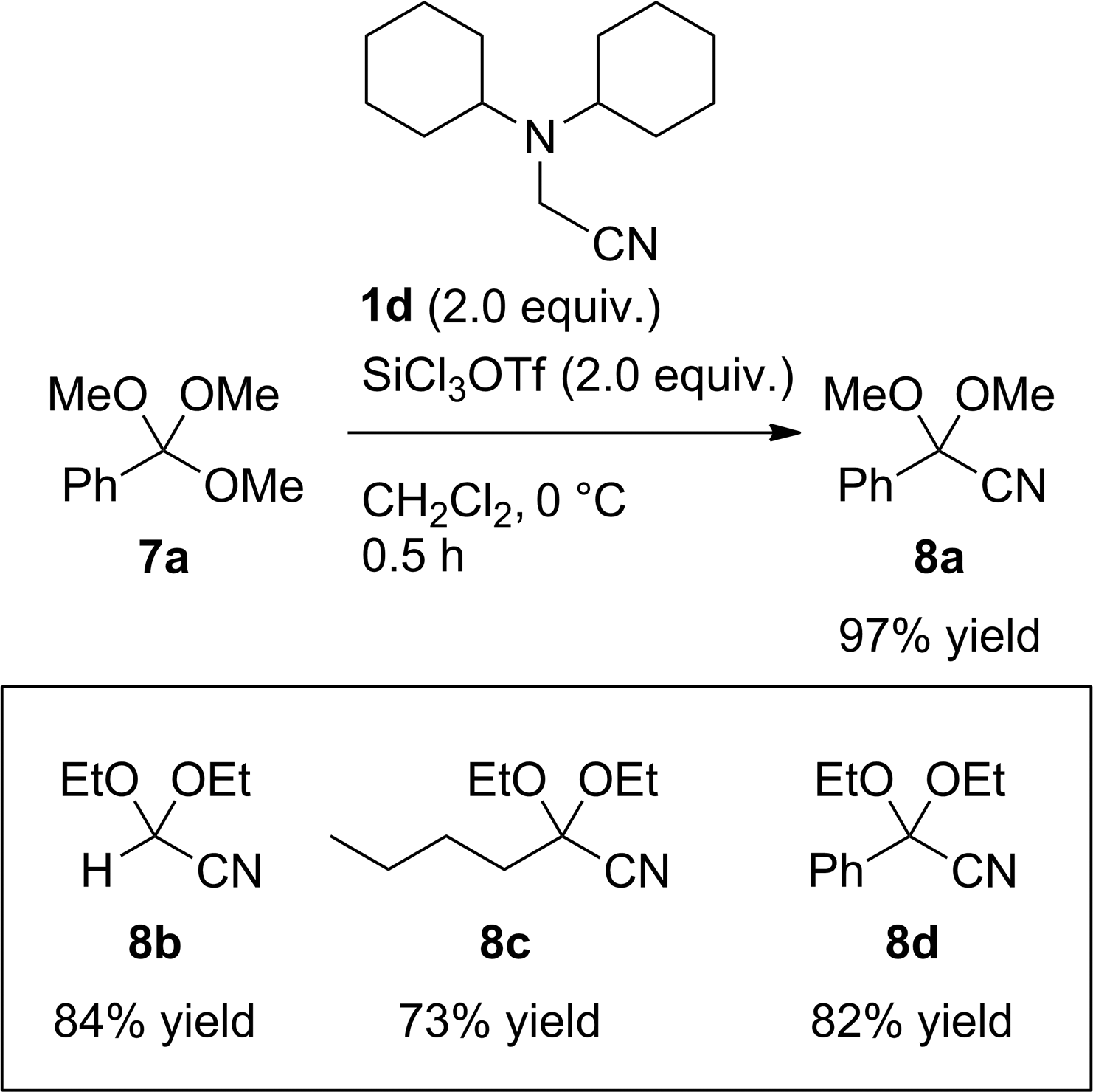
Cyanation of the orthoesters 7. For the preparation of 8c and 8d, cyanoamine 1d (3.0 equiv.) and SiCl3OTf (3.0 equiv.) were used.
CAS number: 425386-60-3
LY-450139, also known as Semagacestat, is a small molecule drug with a maximum clinical trial phase of III and has 1 investigational indication. It was designed to reduce the production of beta-amyloid (Aβ) peptides, which are thought to be involved in the development of Alzheimer's. However, a phase 3 trial was halted early due to the drug's ineffectiveness and potential for harm.
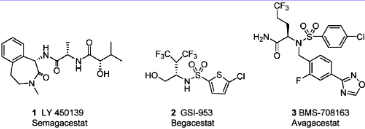
Representative γ-secretase inhibitors: LY-450139, GSI-953, BMS-708163.
CAS number: 4254-14-2
Aliphatic alcohol is a propane-1,2-diol. It has a role as a human metabolite and an Escherichia coli metabolite. It is an enantiomer of a (S)-propane-1,2-diol.
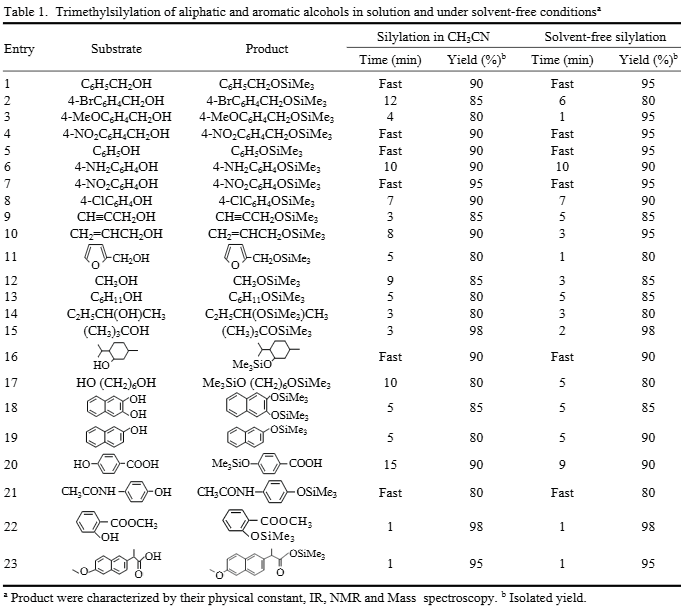
Trimethylsilylation of aliphatic and aromatic alcohols in solution and under solvent-free conditions
CAS number: 429-41-4
Tetrabutylammonium fluoride is a fluoride salt and a tetrabutylammonium salt. It has a role as a phase-transfer catalyst.
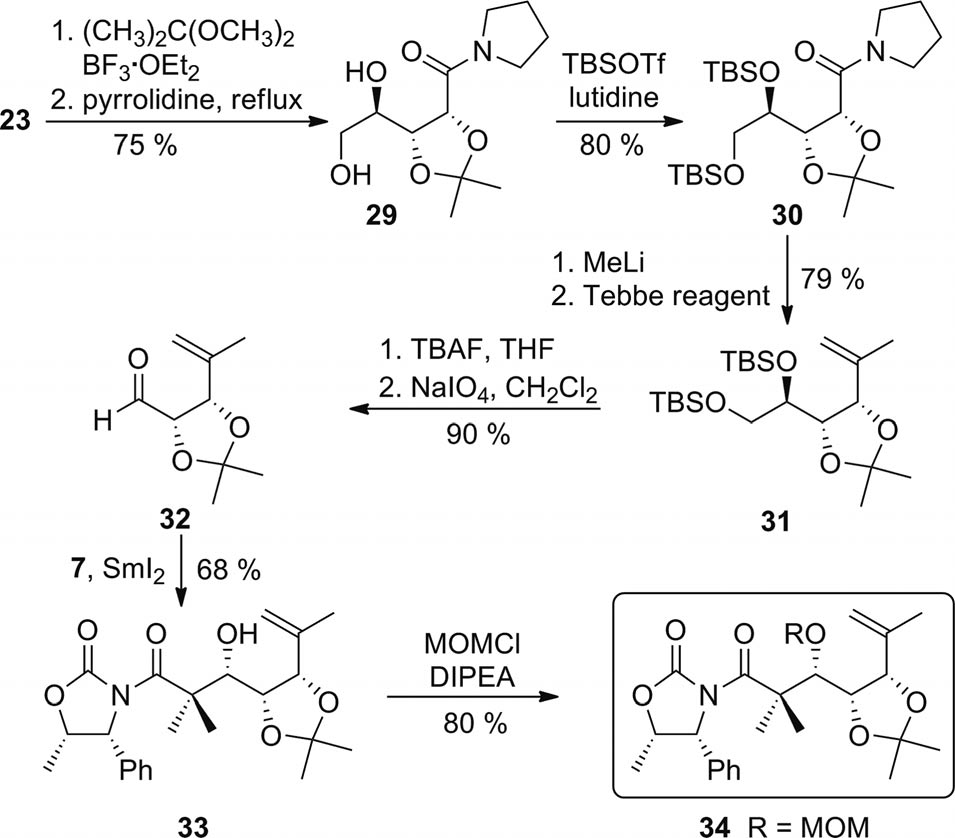
Preparation of alkene 34 (TBAF = tetrabutylammonium fluoride).
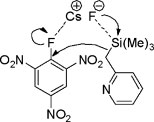
Proposed six membered transition state mechanism
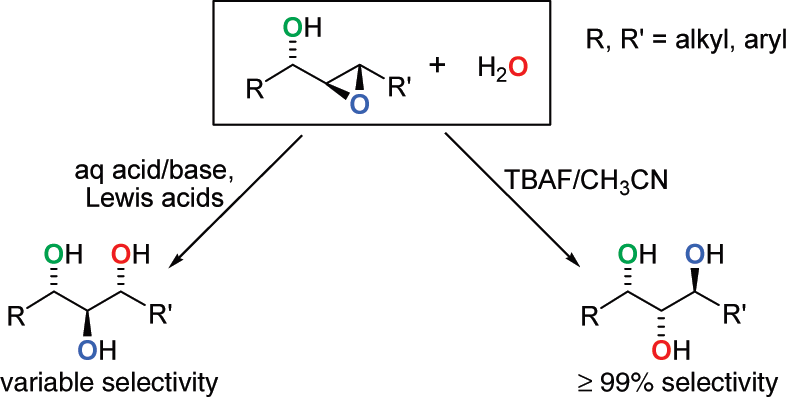
Ring Opening of α,β-Epoxyalcohols Using TBAF Compared to Other Methods
CAS number: 435-97-2
Phenprocoumon is a hydroxycoumarin that is 4-hydroxycoumarin which is substituted at position 3 by a 1-phenylpropyl group. It has a role as an anticoagulant and an EC 1.6.5.2 [NAD(P)H dehydrogenase (quinone)] inhibitor.
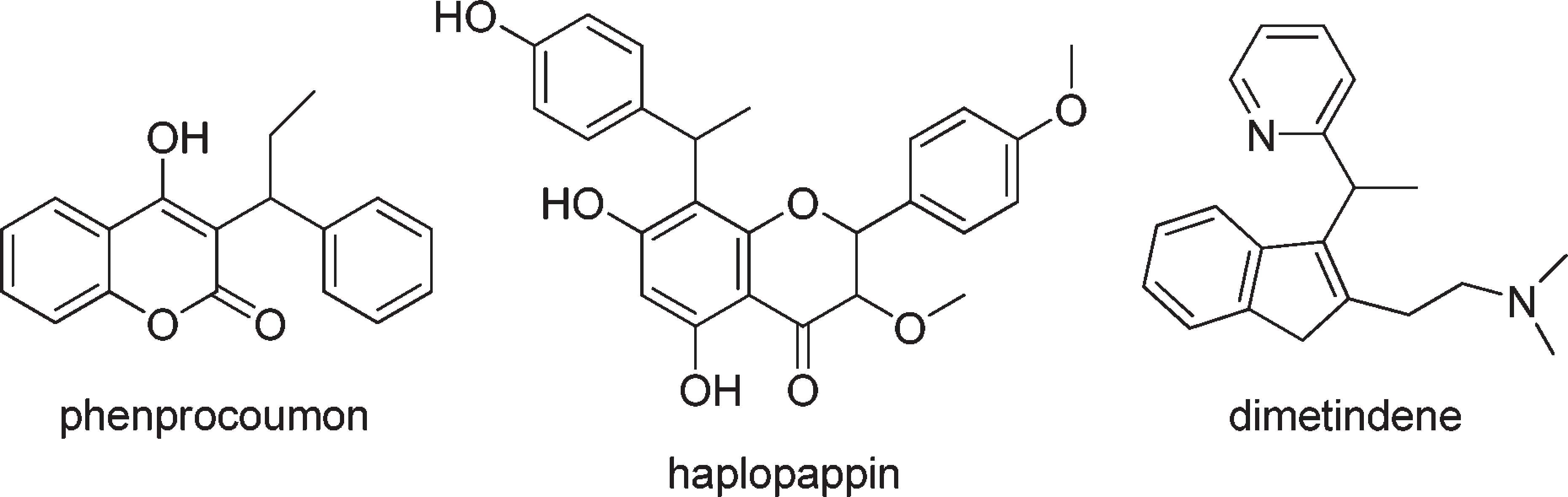
Biologically active compounds and pharmaceutical substrates.
CAS number: 4363-34-2
Vinylboronic acid, also known as ethenylboronic acid, is an organoboronic compound features a vinyl group (–CH=CH₂) directly bonded to a boronic acid moiety, making it a valuable reagent in organic synthesis, particularly in Suzuki–Miyaura cross-coupling reactions. This compound is widely used for forming carbon–carbon bonds, especially in the synthesis of complex molecules such as pharmaceuticals, agrochemicals, and advanced materials. Vinylboronic acid is typically a white to off-white solid, sensitive to moisture and air, and requires proper storage under inert conditions to prevent degradation. Its unique combination of reactivity and functional group tolerance makes it an important building block in modern synthetic chemistry.
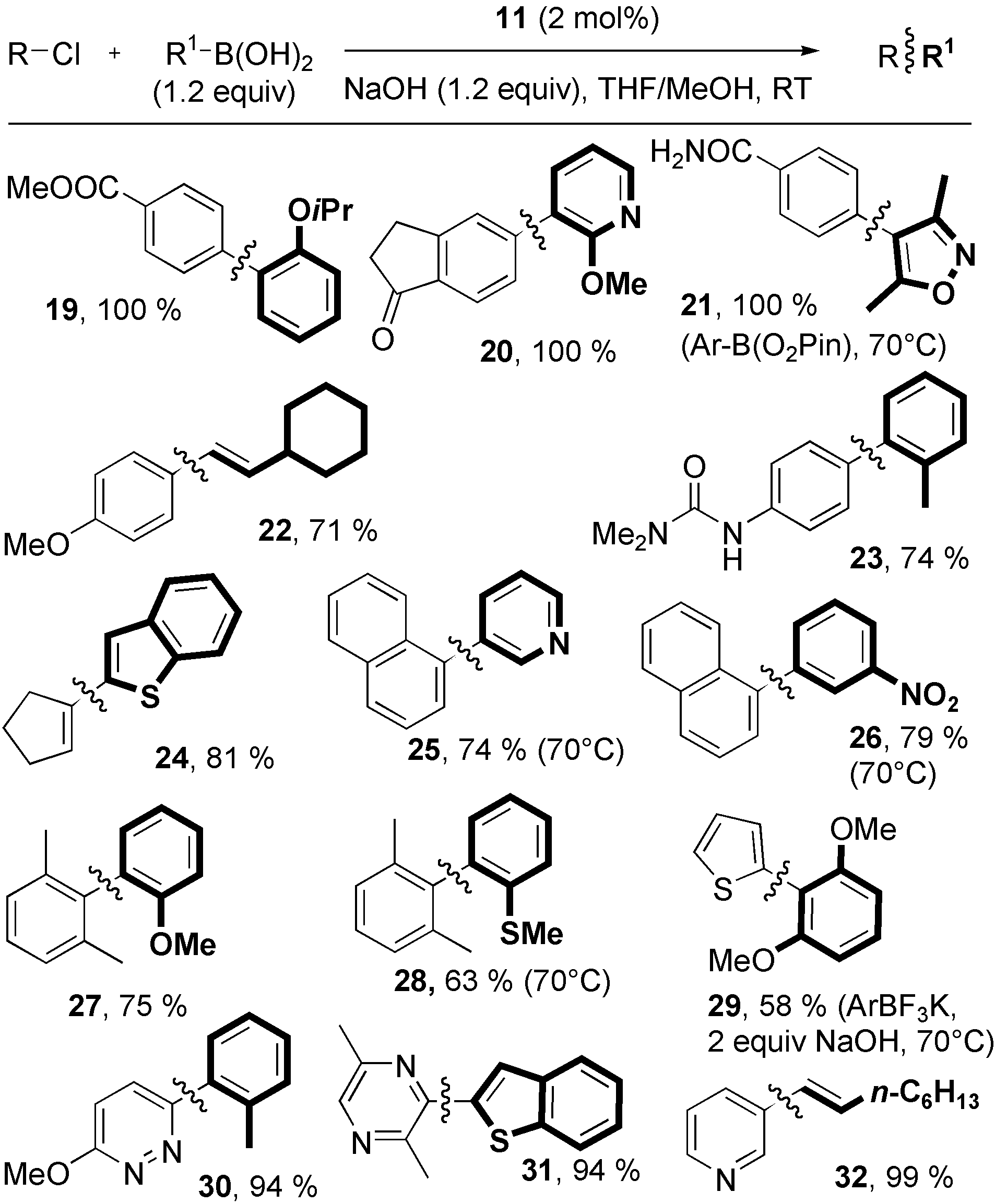
Aryl and vinyl chlorides were cross-coupled with aryl and vinyl boronic acids, pinacol esters, and potassium trifluoroborate under nonbasic conditions mediated by 11. Yields are from isolated chromatographically homogeneous material (average of two runs) and were not optimized for time and catalyst loading.