Vinylboronic acid
CAS number: 4363-34-2
Vinylboronic acid, also known as ethenylboronic acid, is an organoboronic compound features a vinyl group (–CH=CH₂) directly bonded to a boronic acid moiety, making it a valuable reagent in organic synthesis, particularly in Suzuki–Miyaura cross-coupling reactions. This compound is widely used for forming carbon–carbon bonds, especially in the synthesis of complex molecules such as pharmaceuticals, agrochemicals, and advanced materials. Vinylboronic acid is typically a white to off-white solid, sensitive to moisture and air, and requires proper storage under inert conditions to prevent degradation. Its unique combination of reactivity and functional group tolerance makes it an important building block in modern synthetic chemistry.
Related images
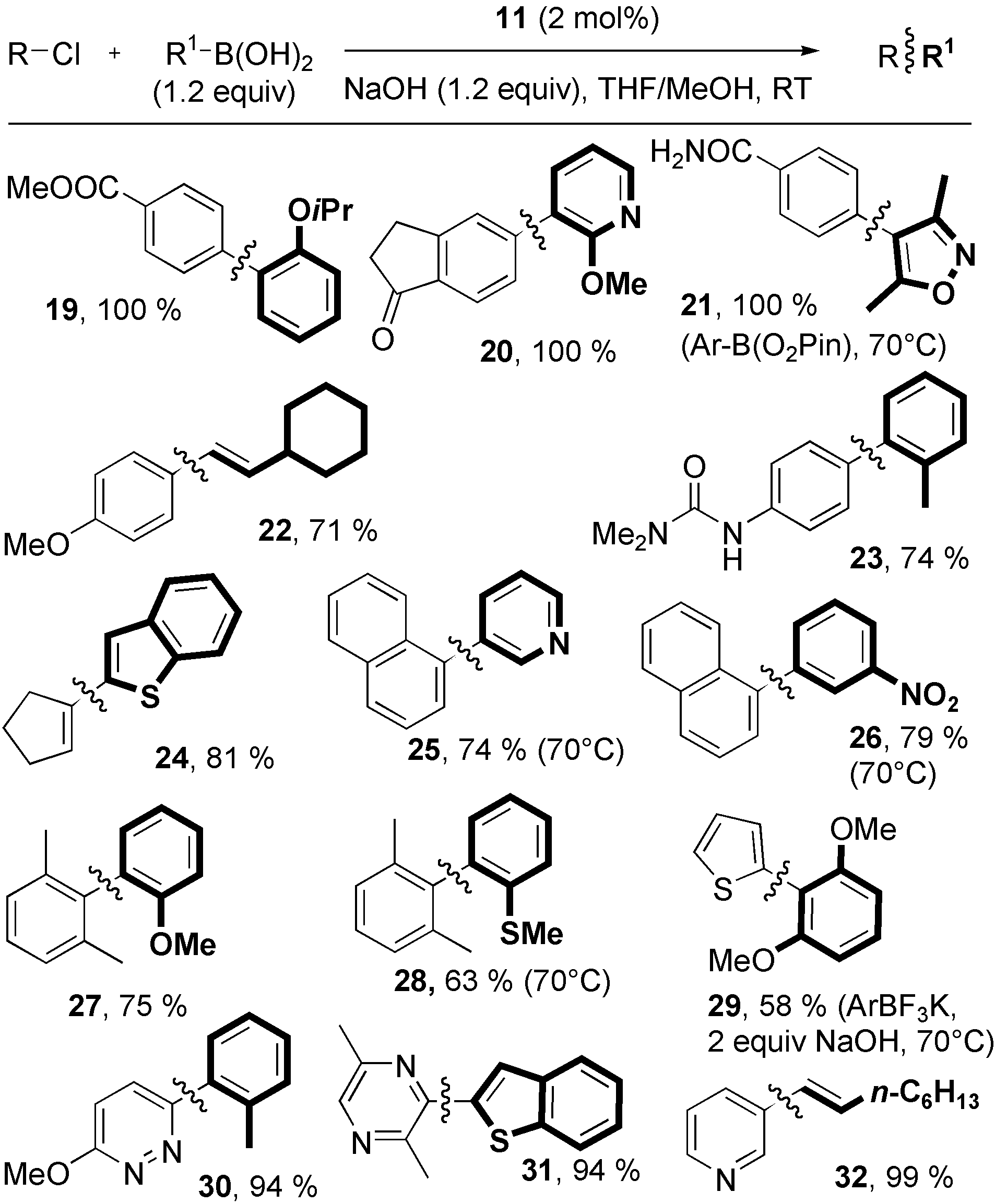
Aryl and vinyl chlorides were cross-coupled with aryl and vinyl boronic acids, pinacol esters, and potassium trifluoroborate under nonbasic conditions mediated by 11. Yields are from isolated chromatographically homogeneous material (average of two runs) and were not optimized for time and catalyst loading.
Related Questions and Answers
No related questions yet