Chemicals list & Research Gallery
CAS number: 4008-48-4
Nitroxoline is an obscure, yet potentially effective and safe antimicrobial agent in uncomplicated lower UTIs.
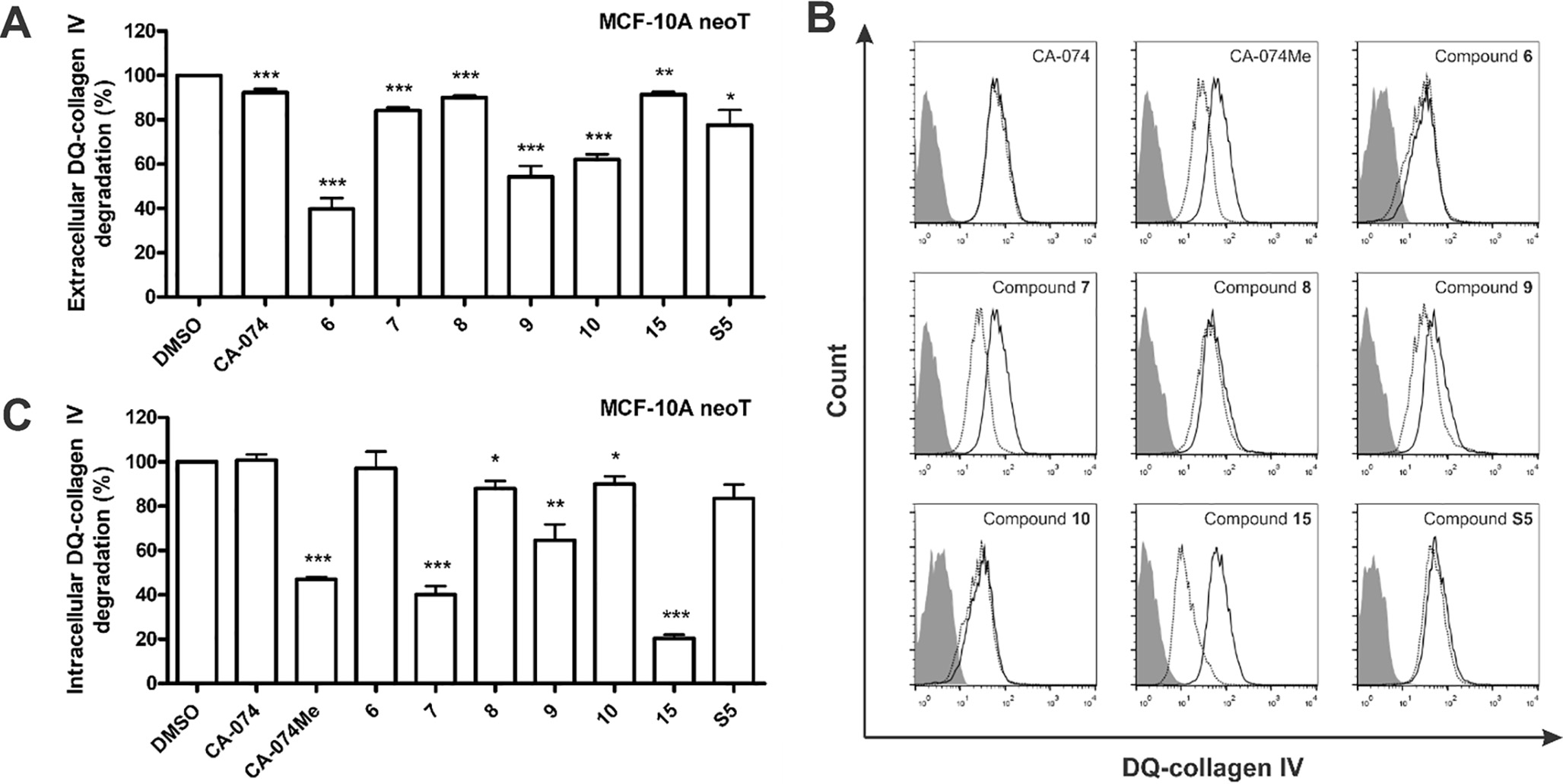
Nitroxoline derivatives inhibit extracellular and intracellular DQ-collagen IV degradation.
CAS number: 4039-32-1
Lithium bis(trimethylsilyl)amide, commonly abbreviated as LiHMDS or LHMDS, is a strong, non-nucleophilic base used extensively in organic synthesis.
![Azide–enolate [3+2] cycloaddition reactions implicated in amide and triazoline synthesis. LDA=lithium diisopropylamide, LHMDS=lithium hexamethyldisilazide.](http://www.wlxkc.cn/picture/1402519_01.png)
Azide–enolate [3+2] cycloaddition reactions implicated in amide and triazoline synthesis. LDA=lithium diisopropylamide, LHMDS=lithium hexamethyldisilazide.
CAS number: 404011-02-5
IC87361, chemically known as 5-hydroxy-7-morpholin-4-yl-2-phenyl-4H-chromen-4-one (Molecular Formula: C₁₉H₁₇NO₄), is a potent and selective small-molecule inhibitor targeting DNA-dependent protein kinase (DNA-PK), with an IC₅₀ of approximately 34 nM and over 50-fold selectivity compared to p110β (a PI3K isoform). Functionally, IC87361 acts as a radiosensitizer: it enhances the sensitivity of wild-type endothelial cells and significantly potentiates the effects of ionizing radiation on tumor microvasculature, particularly when administered at ~10 µM for at least 4 hours.
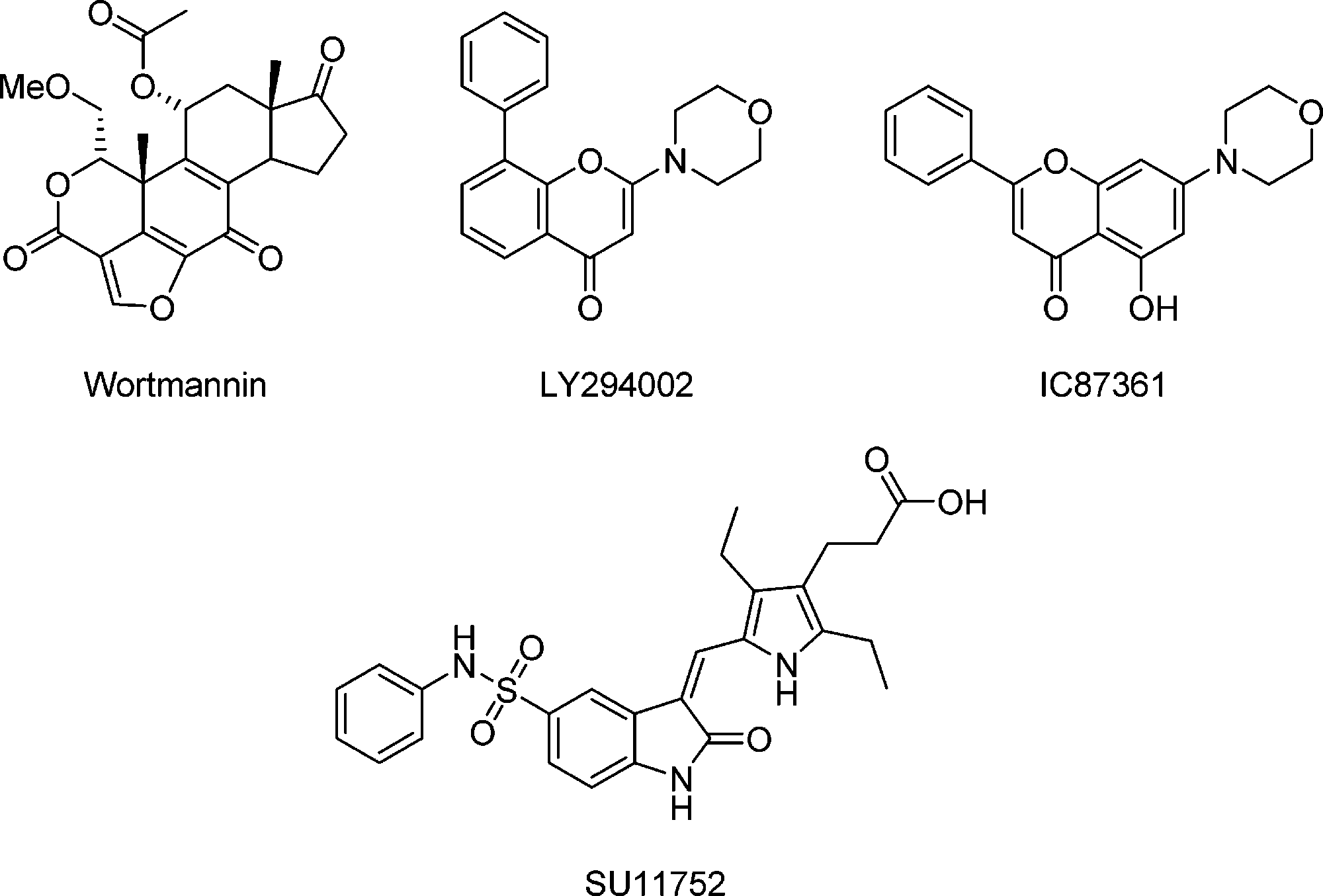
Structures of DNA-PK Inhibitors: wortmannin, LY294002, IC87361, SU11752.
CAS number: 404950-80-7
Panobinostat is an oral deacetylace (DAC) inhibitor approved on February 23, 2015 by the FDA for the treatment of multiple myeloma. The approval was accelerated based on progression-free survival, therefore confirmatory trials by the sponsor to demonstrate clinical efficacy in multiple myeloma treatment are in progress of being conducted. Panobinostat is marketed by Novartis under the brand name Farydak. Panobinostat acts as a non-selective histone deacetylase inhibitor (pan-HDAC inhibitor) and it is the most potent DAC inhibiting agent available on the market.
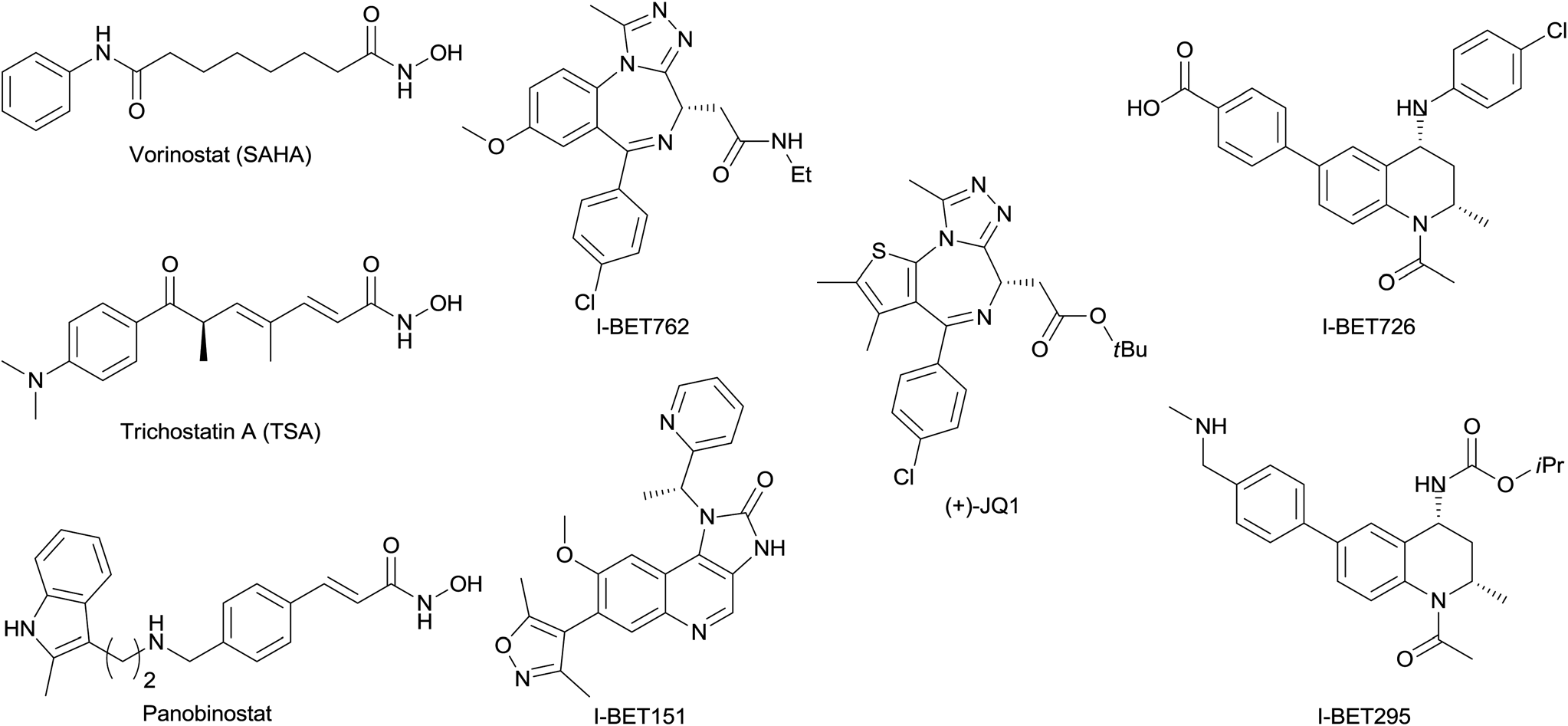
Structures of selected published BET inhibitors and selected hydroxamic acid HDAC inhibitors.
CAS number: 4054-38-0
1,3-Cycloheptadiene is a cyclic molecule with a seven-membered ring containing two carbon-carbon double bonds (alkene groups) in a 1,3-relationship. It's also known as cyclohepta-1,3-diene.
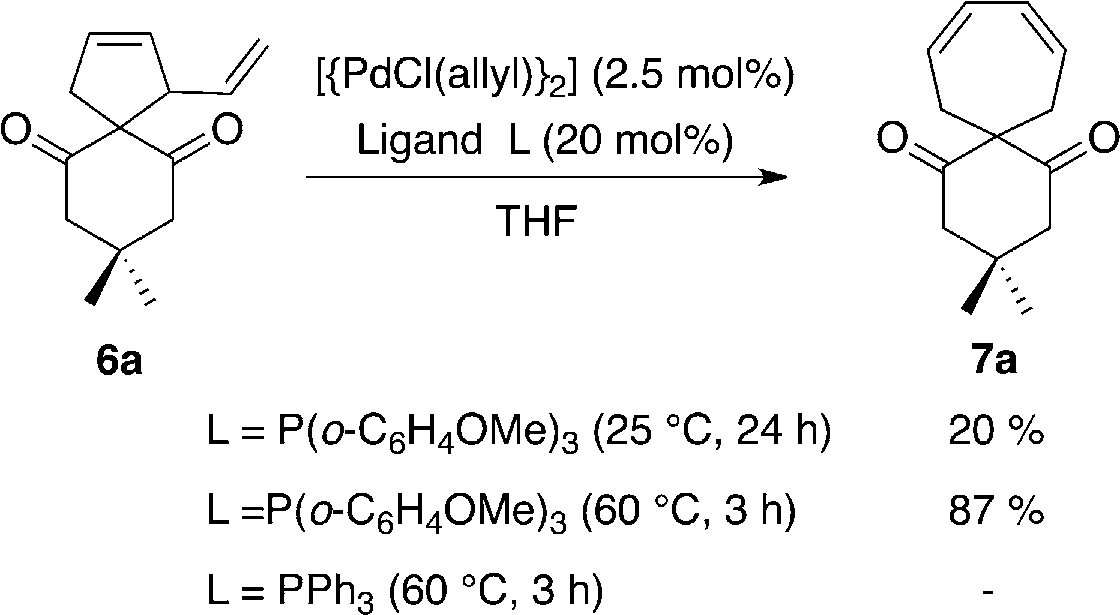
Transformation of vinylcyclopentene 6a into cycloheptadiene 7a.
CAS number: 407-41-0
Phosphoserine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655).
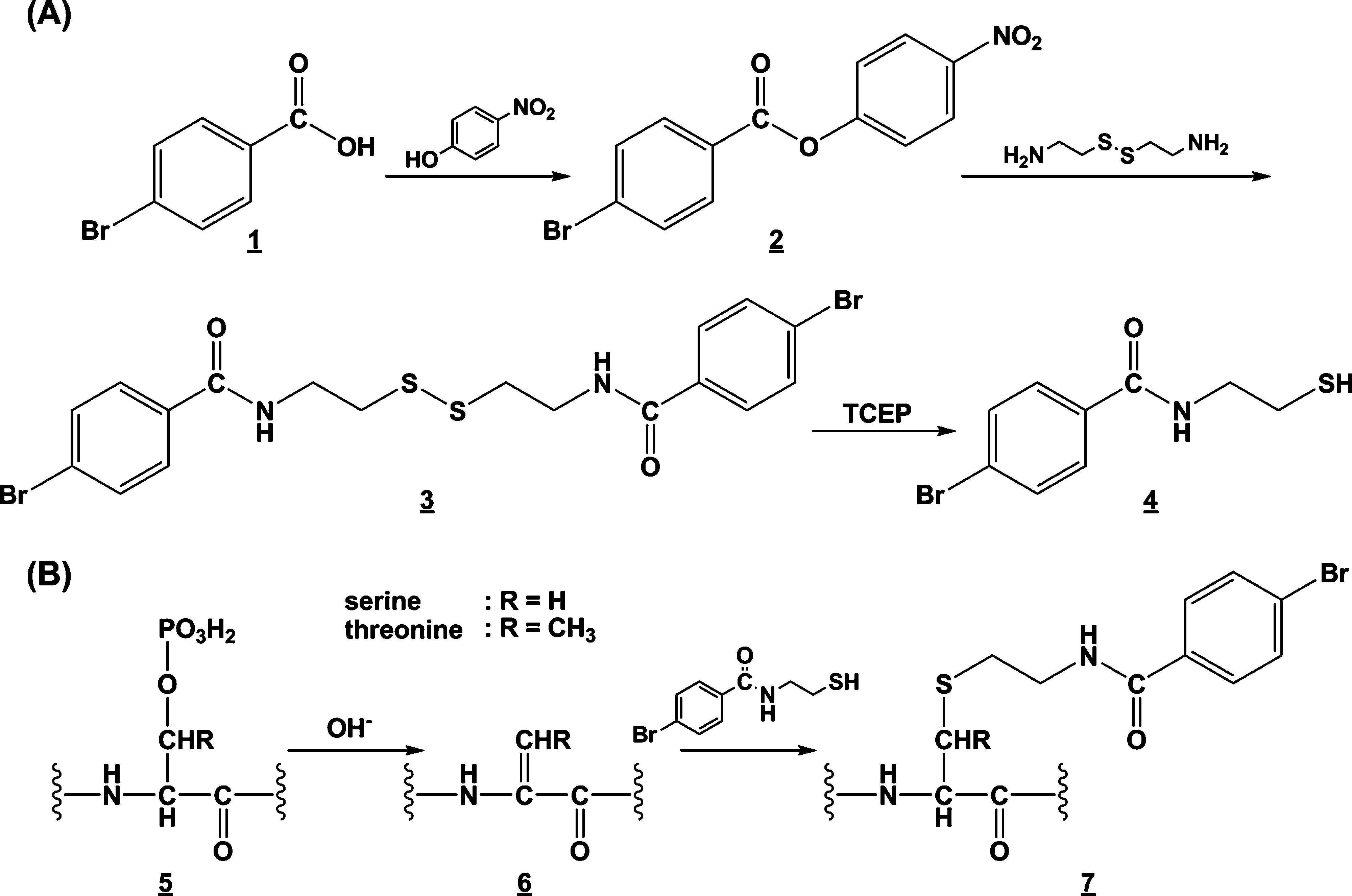
Synthesis of N-(4-bromobenzoyl)aminoethanethiol (A) and derivatization of phosphoserine and phosphothreonine residues (B).
CAS number: 4083-64-1
Tosyl isocyanate, also known as p-toluenesulfonyl isocyanate, is a colorless liquid that reacts readily with water and other protic solvents. It is commonly used as a reagent in organic synthesis, particularly in the introduction of the sulfonyl isocyanate group.
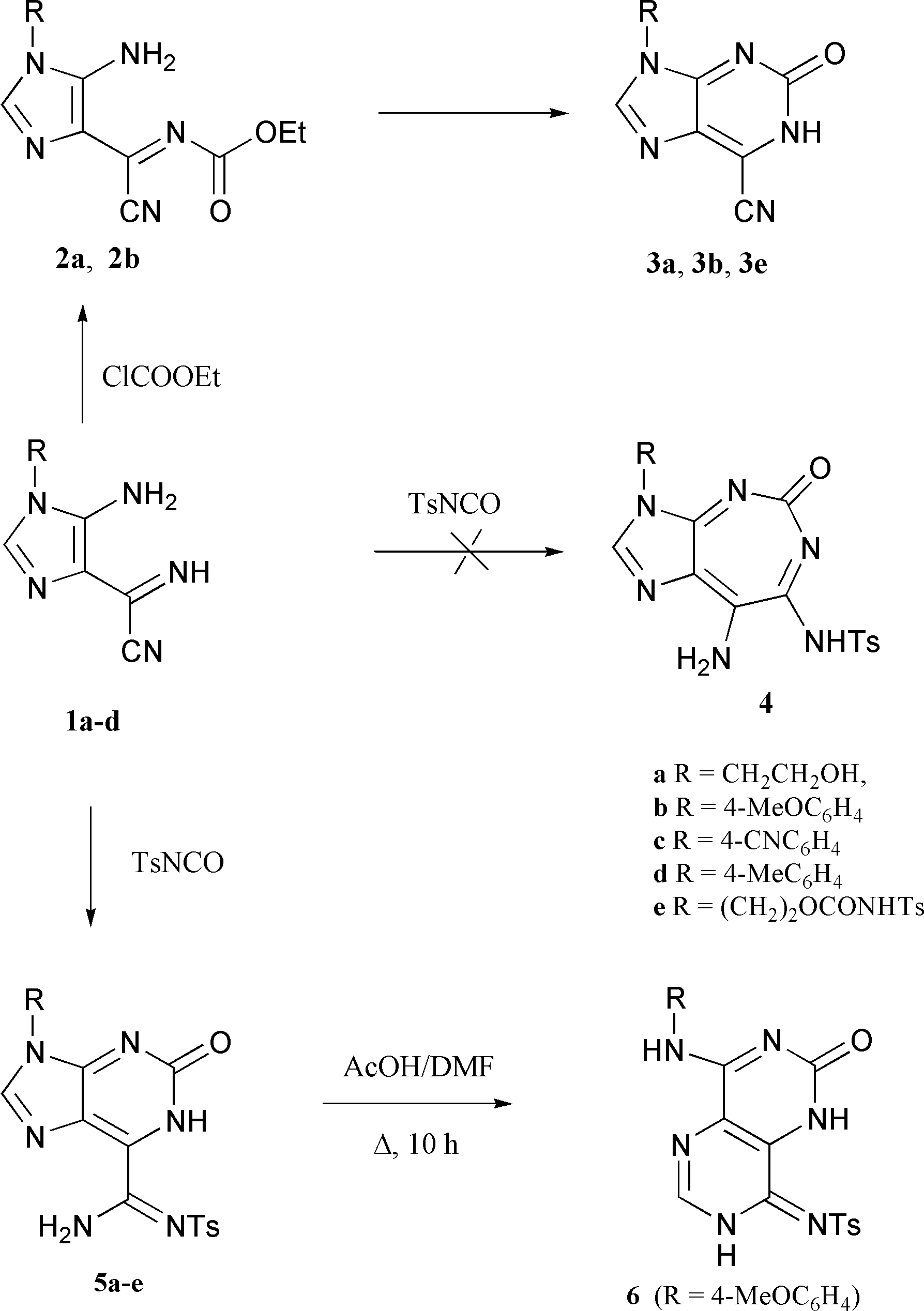
The reaction of imidazole 1 with a slight excess of tosyl isocyanate was carried out under a nitrogen atmosphere. The reagents were combined at 0 ЊC and the mixture was stirred at room temperature for a few hours. The products 5a–e precipitated from the reaction mixture as yellow solid materials and were isolated by filtration in 85–99% yield (Scheme 1).
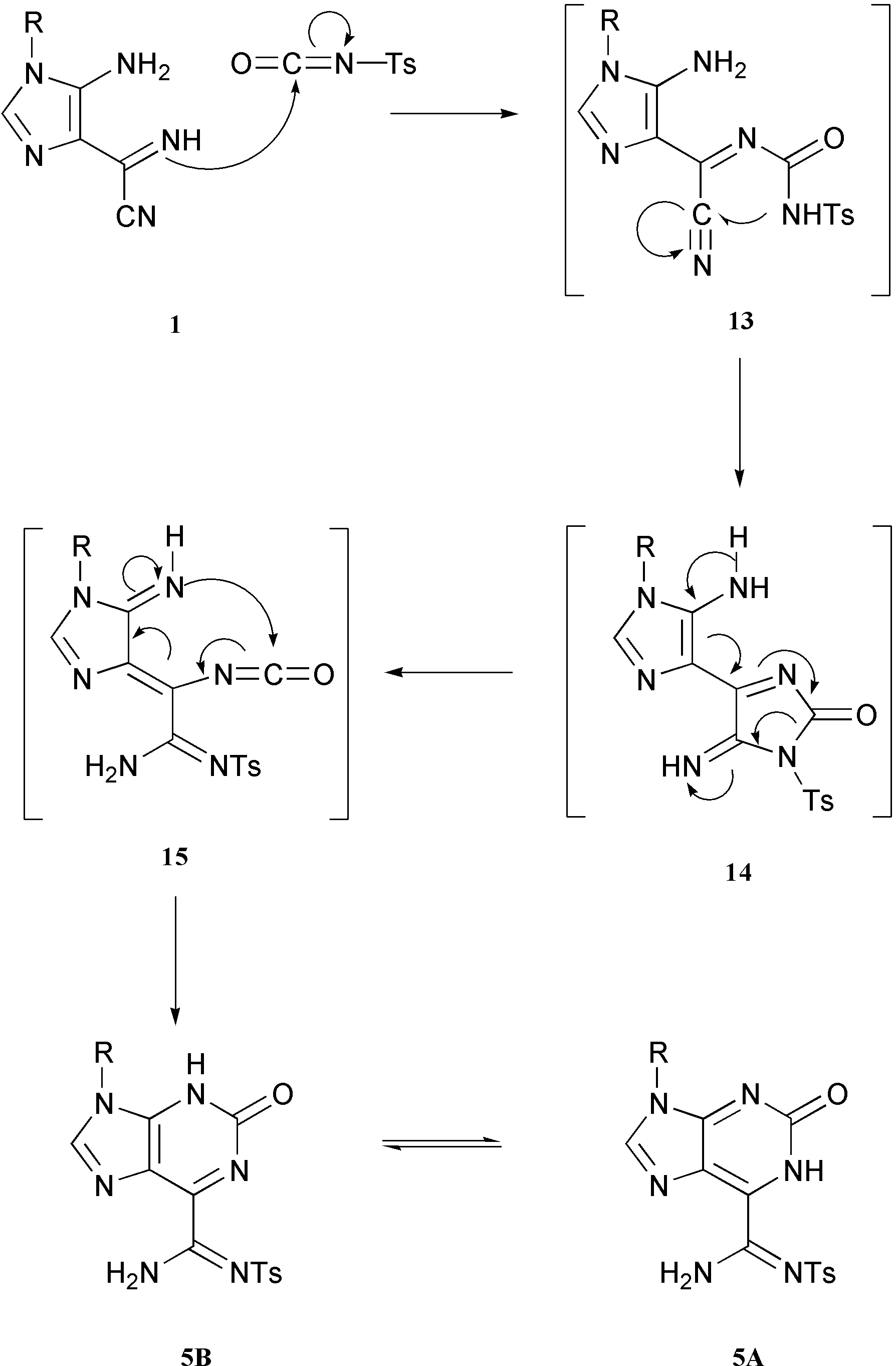
The preferred pathway for the formation of purine 5 in the reaction of imidazole 1 with tosyl isocyanate, must be the one represented in Scheme 5.
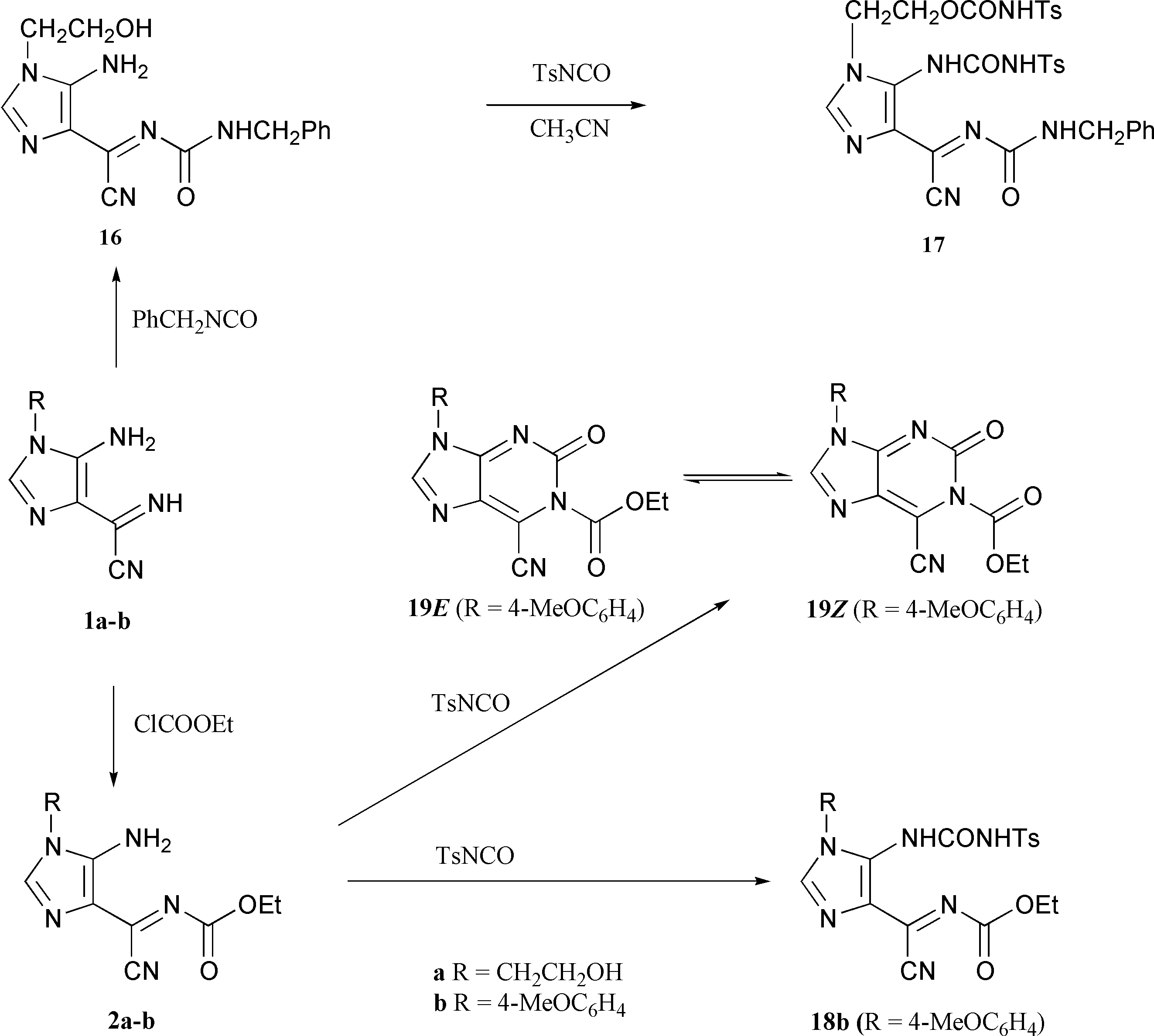
Use of imidazoles 2 and 16 in the reaction with tosyl isocyanate (Scheme 6).
CAS number: 4111-54-0
Lithium diisopropylamide (LDA) is a prominent reagent used in organic synthesis. It is a hindered non-nucleophilic strong base that abstracts hydrogen from active carbon.

Catalytic asymmetric synthesis of (S)-verapamil. LDA=lithium diisopropylamide.
![Azide–enolate [3+2] cycloaddition reactions implicated in amide and triazoline synthesis. LDA=lithium diisopropylamide, LHMDS=lithium hexamethyldisilazide.](http://www.wlxkc.cn/picture/1402519_01.png)
Azide–enolate [3+2] cycloaddition reactions implicated in amide and triazoline synthesis. LDA=lithium diisopropylamide, LHMDS=lithium hexamethyldisilazide.
CAS number: 41340-25-4
Etodolac is a non-steroidal anti-inflammatory drug (NSAID) with anti-inflammatory, analgesic and antipyretic properties. Its therapeutic effects are due to its ability to inhibit prostaglandin synthesis. It is indicated for relief of signs and symptoms of rheumatoid arthritis and osteoarthritis.
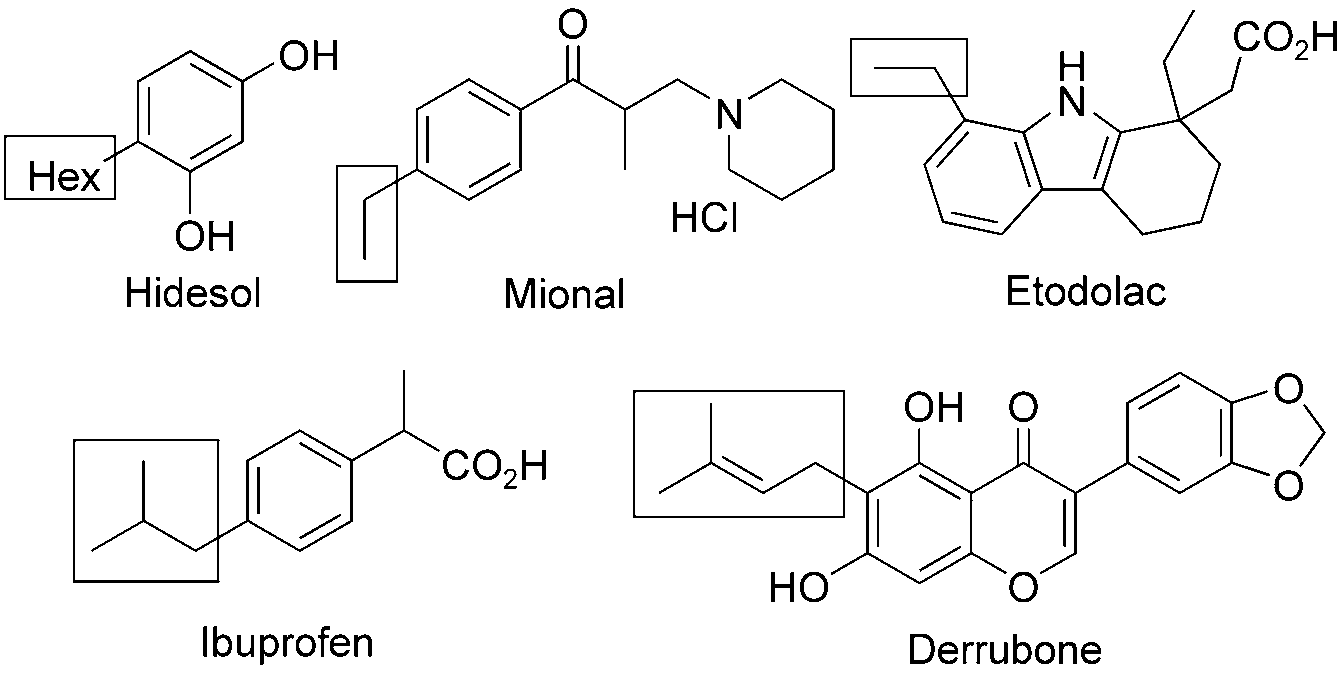
Drugs and bioactive natural products with alkyl side-chains: Hidesol, Mional, Etodolac, Derrubone
CAS number: 41372-20-7
Apomorphine hydrochloride hemihydrate is a medication, specifically a dopamine agonist, used primarily in the treatment of Parkinson's disease and, in some cases, erectile dysfunction.
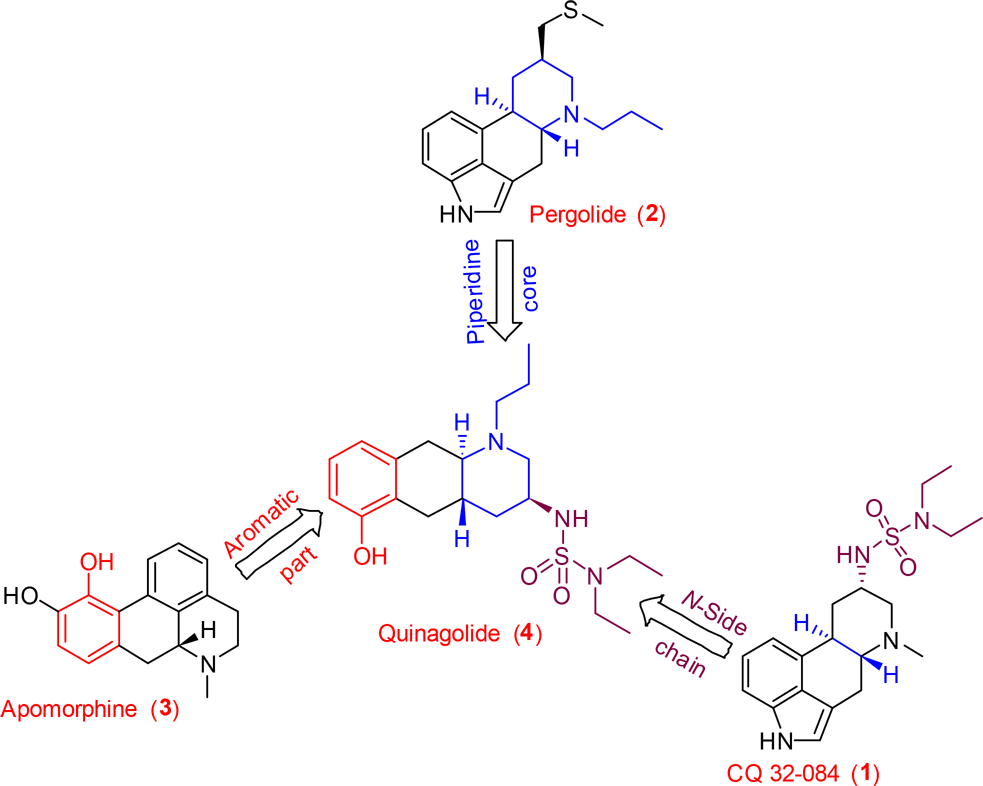
Quinolide (4) has structural features of both ergoline and apomorphine.