Chemicals list & Research Gallery
CAS number: 32315-10-9
Triphosgene, also known as bis(trichloromethyl) carbonate, is a chemical compound used as a reagent in organic synthesis, particularly as a safer alternative to the toxic gas phosgene. It's a white, crystalline solid that decomposes to release phosgene at elevated temperatures. This makes it a convenient and safer way to introduce carbonyl groups into molecules.
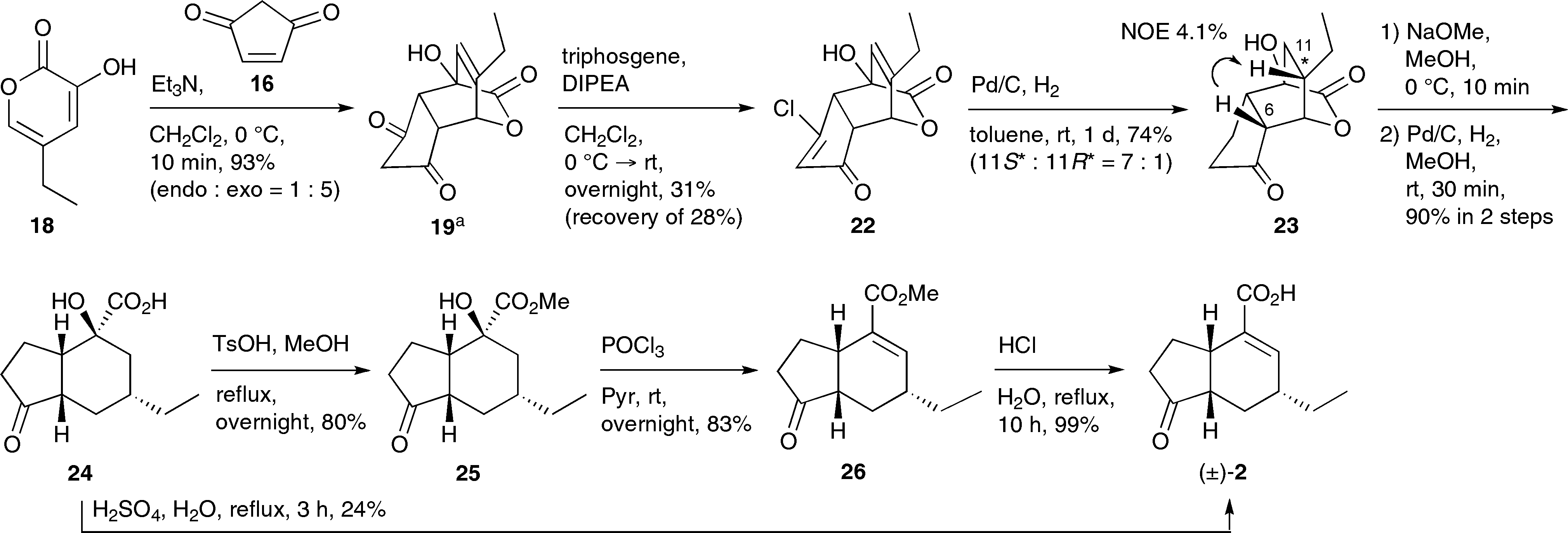
Synthesis of coronafacic acid ( )-2. The product was obtained as a Et3N salt.
CAS number: 32601-90-4
2,2-dibromomethylquinoxaline is a organic chemical compound and a quinoxaline derivative used as a synthetic intermediate in the creation of more complex molecules.
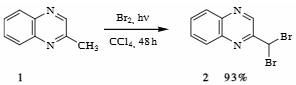
Synthesis of 2,2-dibromomethylquinoxaline 2.
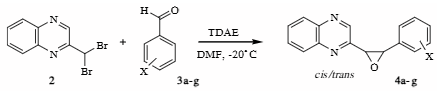
Reactions of 2,2-dibromomethylquinoxaline 2 with aromatic aldehydes in the presence of TDAE.
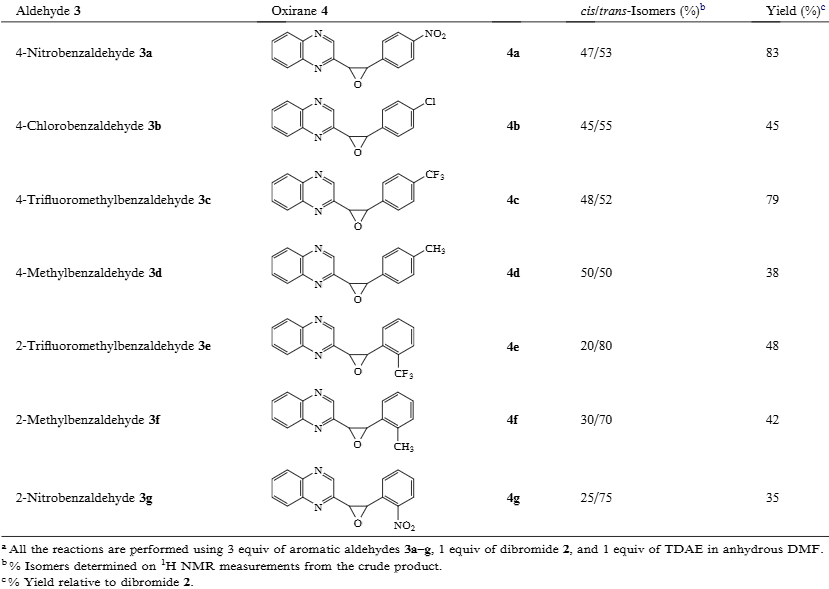
Reactions of 2,2-dibromomethylquinoxaline 2 and aldehydes using TDAE
CAS number: 32645-65-1
Thymidine glycol is a pyrimidine 2'-deoxyribonucleoside.
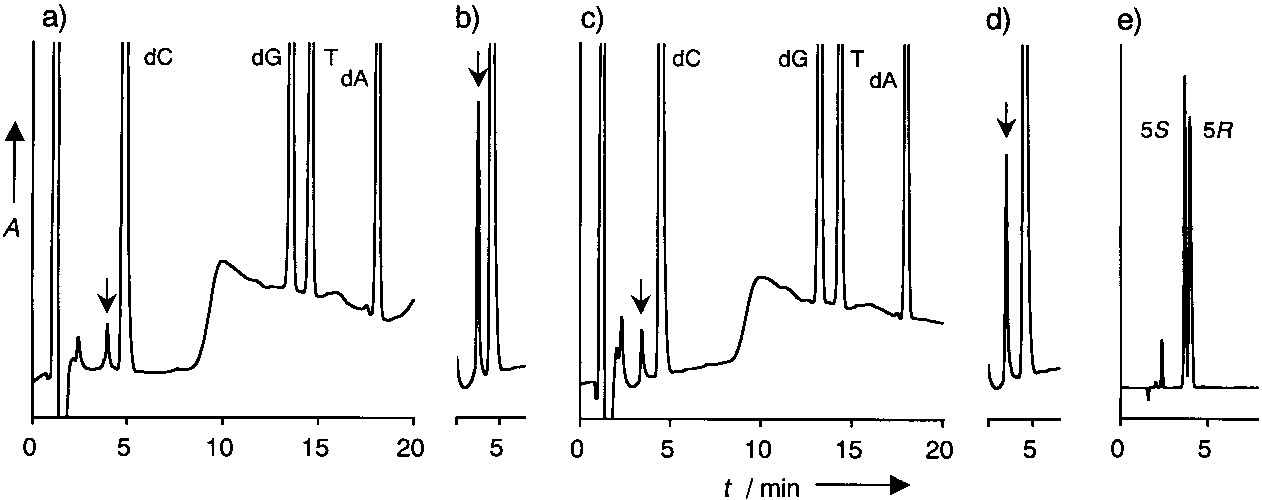
Detection of thymidine glycol after enzyme digestion.
CAS number: 32765-69-8
Triglycerides is fats composed of three fatty acid chains linked to a glycerol molecule.
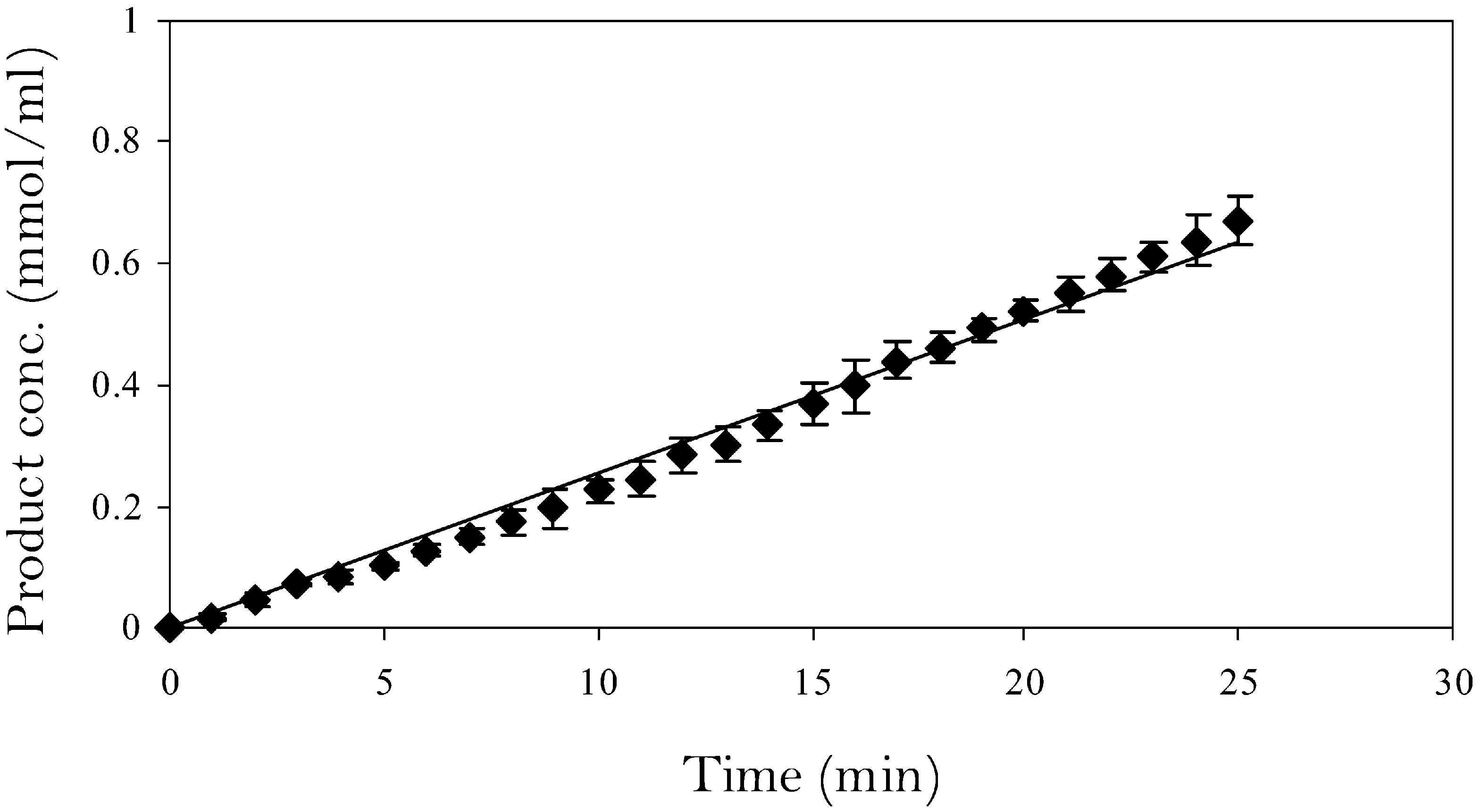
Native catalytic performance of free lipase in the stirred tank reactor with triglycerides as substrate.
CAS number: 32817-12-2
Mycosamine is a mannosamine and a deoxymannose derivative.
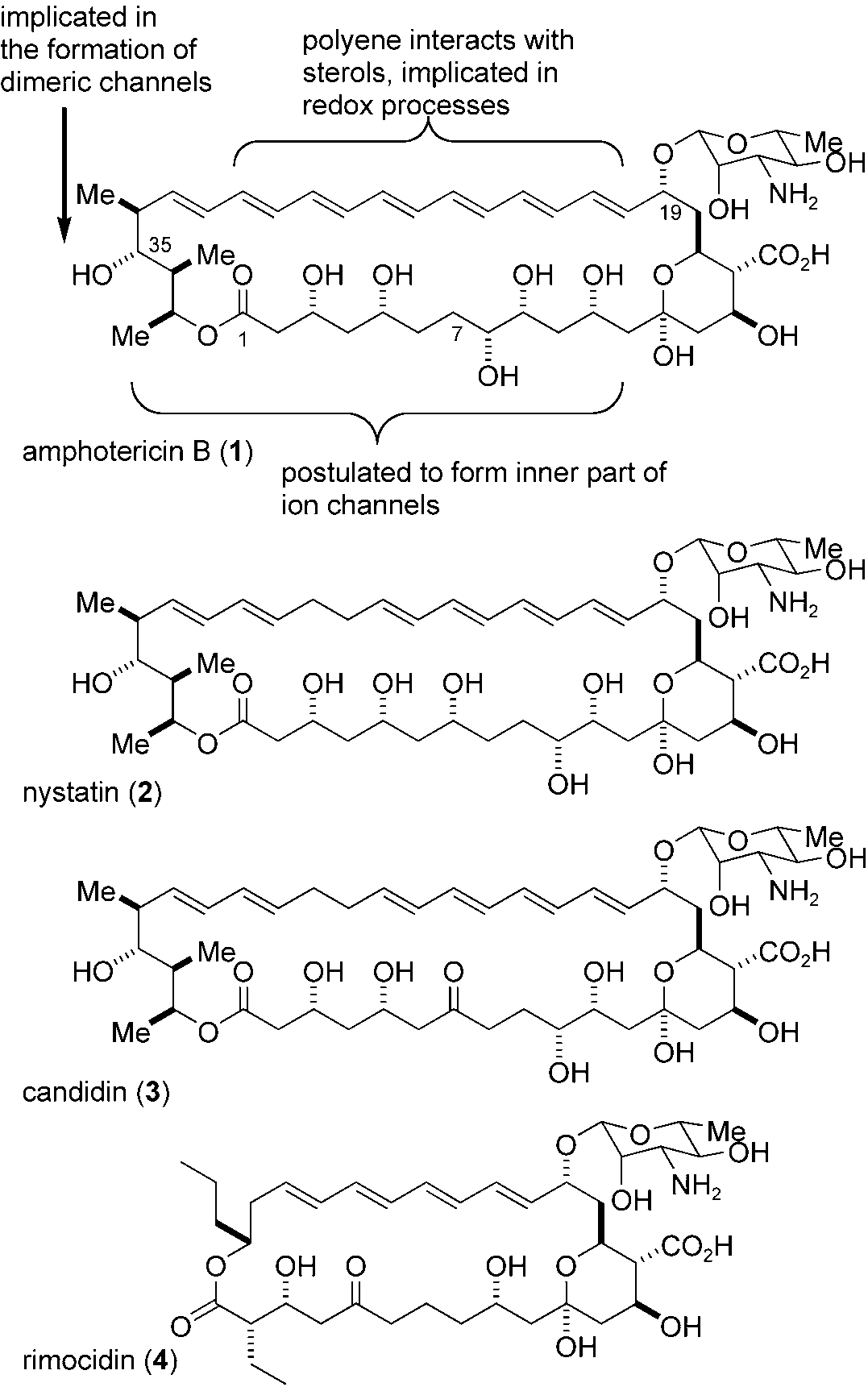
Structure of amphotericin B (1) and related mycosamine polyketides.
CAS number: 33023-08-4
Vincorine is a complex akuammiline alkaloid with a challenging tetracyclic core structure containing a strained seven-membered azepanyl ring and a pyrroloindoline motif. It has been a target for total synthesis due to its potential biological activity.
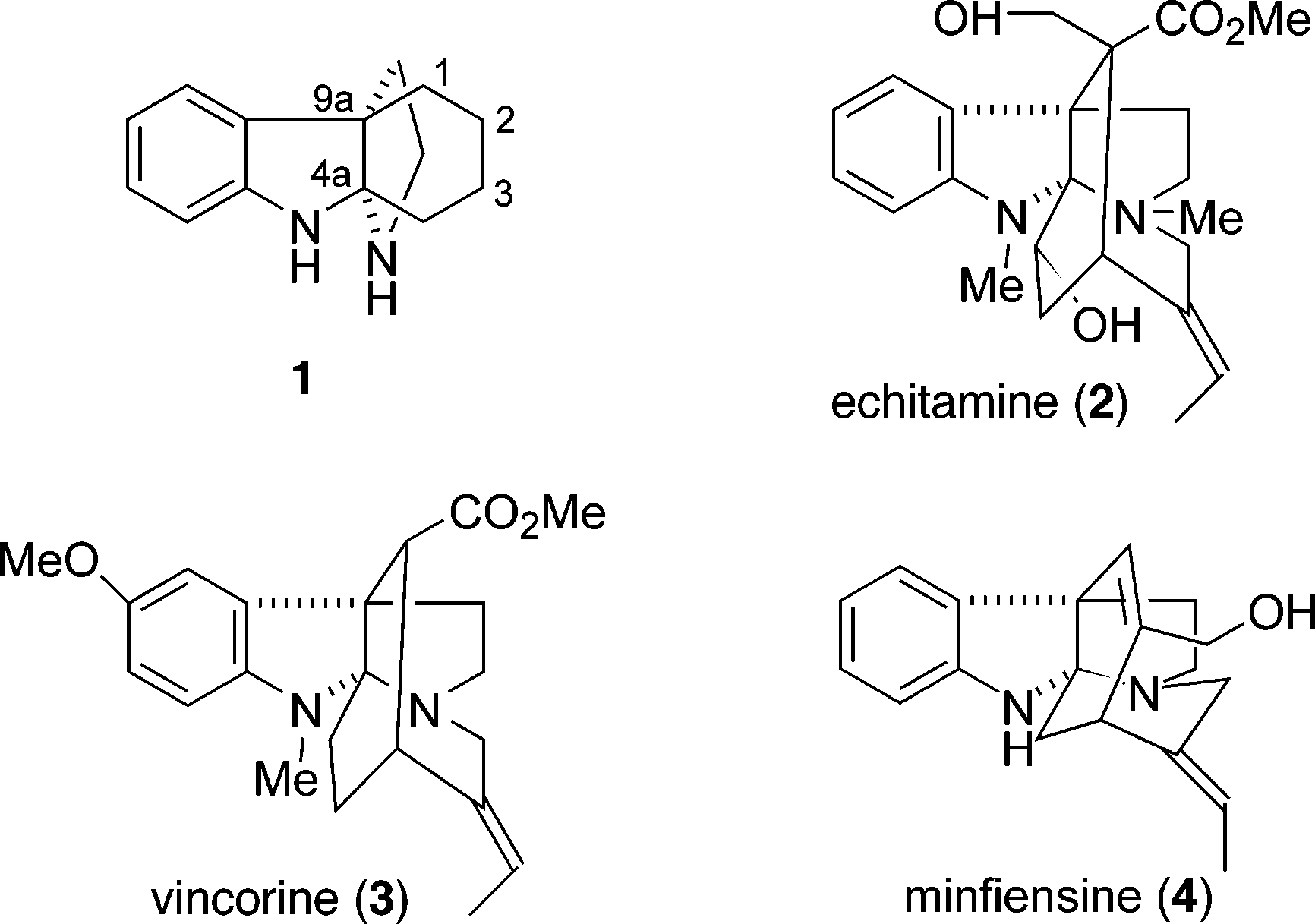
Representative alkaloids containing a 1,2,3,4-tetrahydro-9a,4a-(iminoethano)-9H-carbazole ring system: Echitamine, Vincorine, Minfiensine.
CAS number: 33069-62-4
Paclitaxel is a tetracyclic diterpenoid isolated originally from the bark of the Pacific yew tree, Taxus brevifolia. It is a mitotic inhibitor used in cancer chemotherapy. Note that the use of the former generic name 'taxol' is now limited, as Taxol is a registered trade mark. It has a role as a microtubule-stabilising agent, a metabolite, a human metabolite and an antineoplastic agent. It is a tetracyclic diterpenoid and a taxane diterpenoid. It is functionally related to a baccatin III.
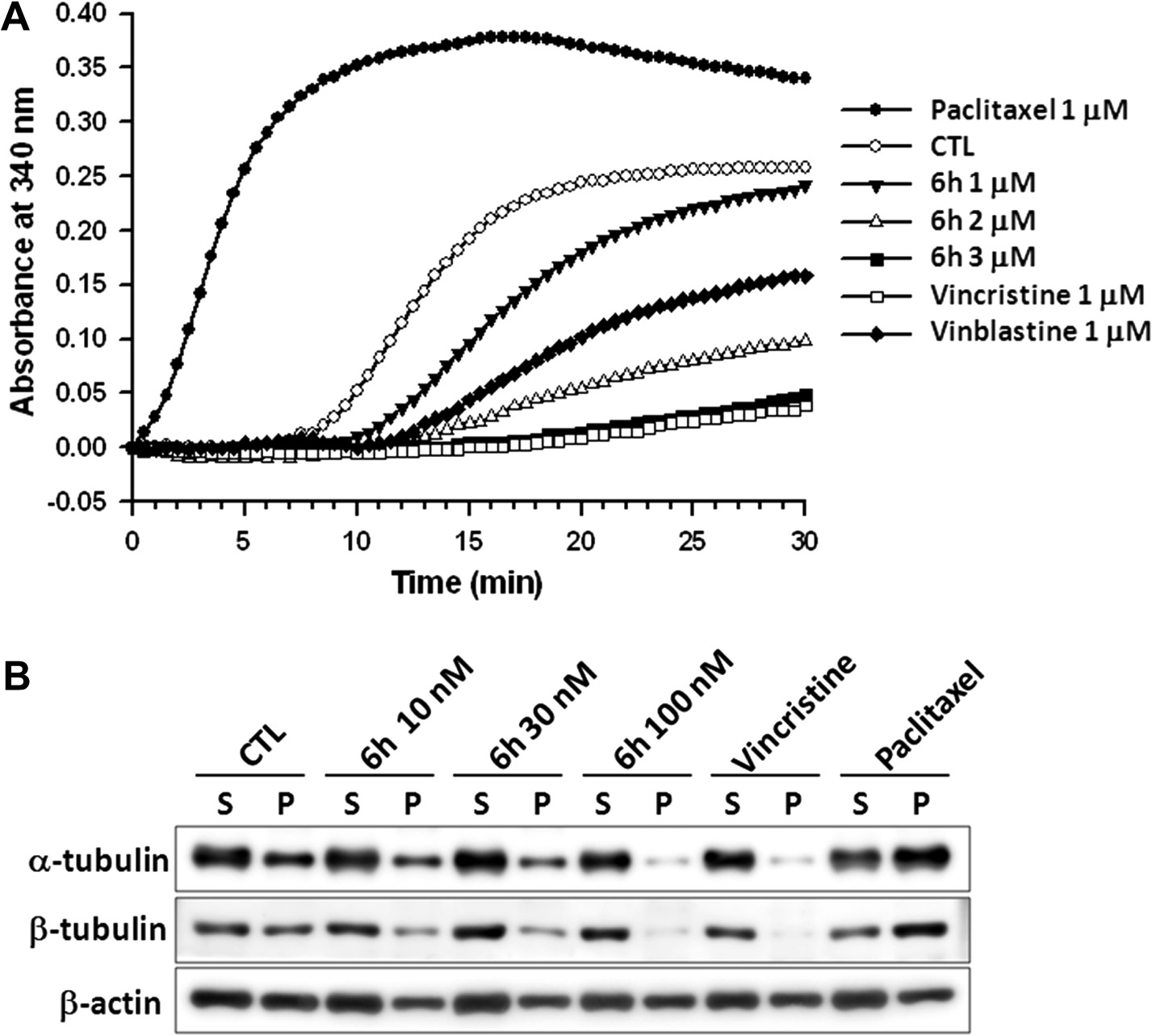
Action mechanism of 6h for anti-proliferation of NCI-H522 non-small cell lung cancer cells. (A) Compound 6h, vincristine, vinblastine, and paclitaxel were incubated with pure tubulin proteins in GPEM buffer. Alternation of tubulin assembly was recorded at absorbance 340 nm. (B) Cells were treated with vehicle (DMSO, as control), 6h (10, 30, and 100 nM), 100 nM vincristine, and 100 nM paclitaxel for 24 h. Cytosolic (S, soluble) and cytoskeletal (P, polymerized tubulin) fractions were separated and followed by Western blot analysis for detection of a-tubulin, b-tubulin, and b-actin protein expression.
CAS number: 3317-67-7
Cobalt phthalocyanine (CoPc) is a chemical compound consisting of a cobalt ion at the center of a phthalocyanine ligand, which is a large, aromatic ring structure. It's a type of metal phthalocyanine known for its brilliant blue color and various applications. CoPc is particularly recognized for its catalytic properties, especially in electrocatalytic reactions.
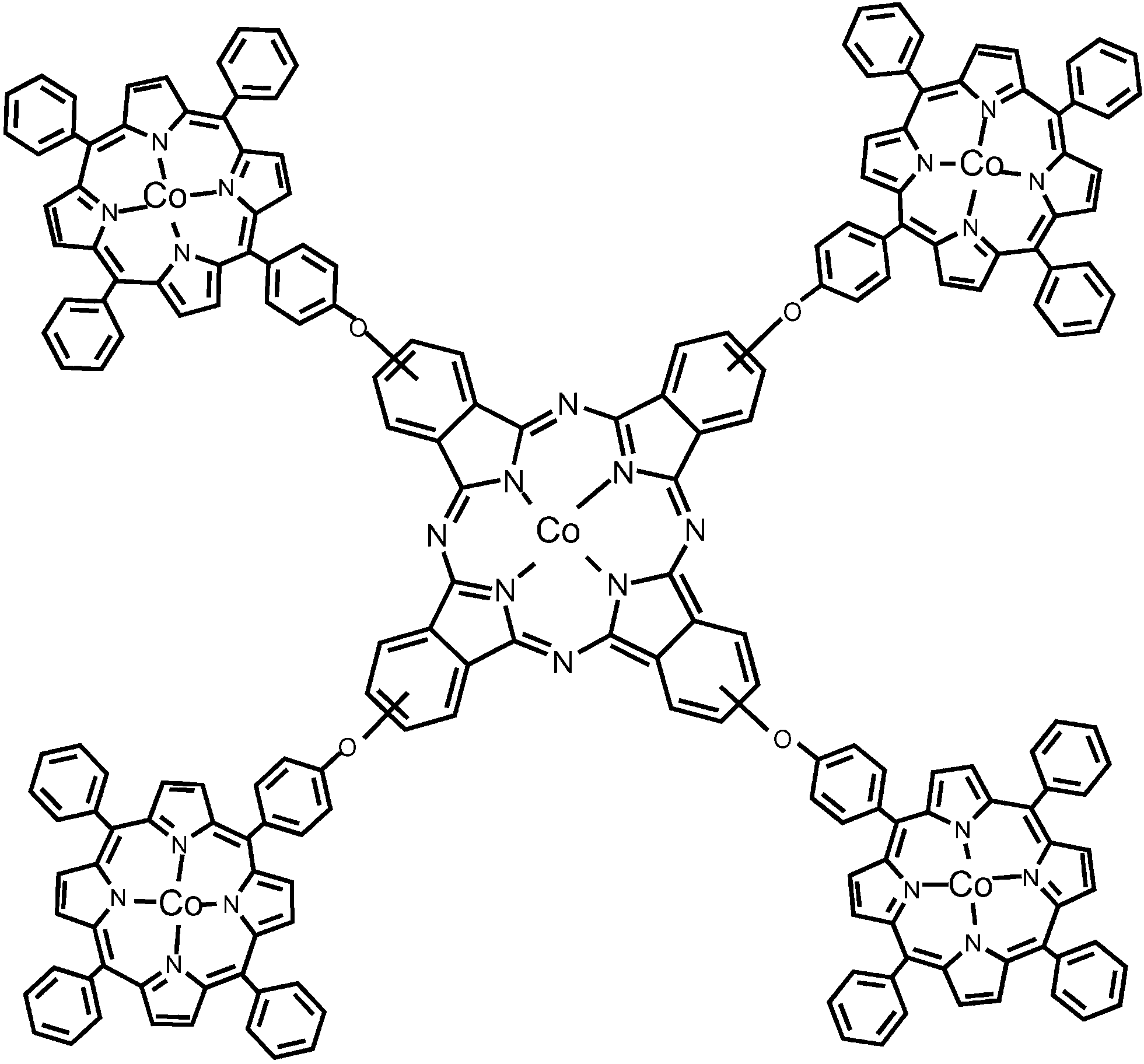
The molecular structure of a cobalt phthalocyanine–cobalt porphyrin CoPc–(CoTPP)4 pentamer.
CAS number: 334-88-3
Diazomethane, a yellow gas normally used as a solution in ether, readily esterifies fatty acids in the presence of methanol at room temperature. It is a strong respiratory irritant. Diazomethane is usually prepared by the decomposition of various derivatives of N-methyl-N-nitrosoamines.
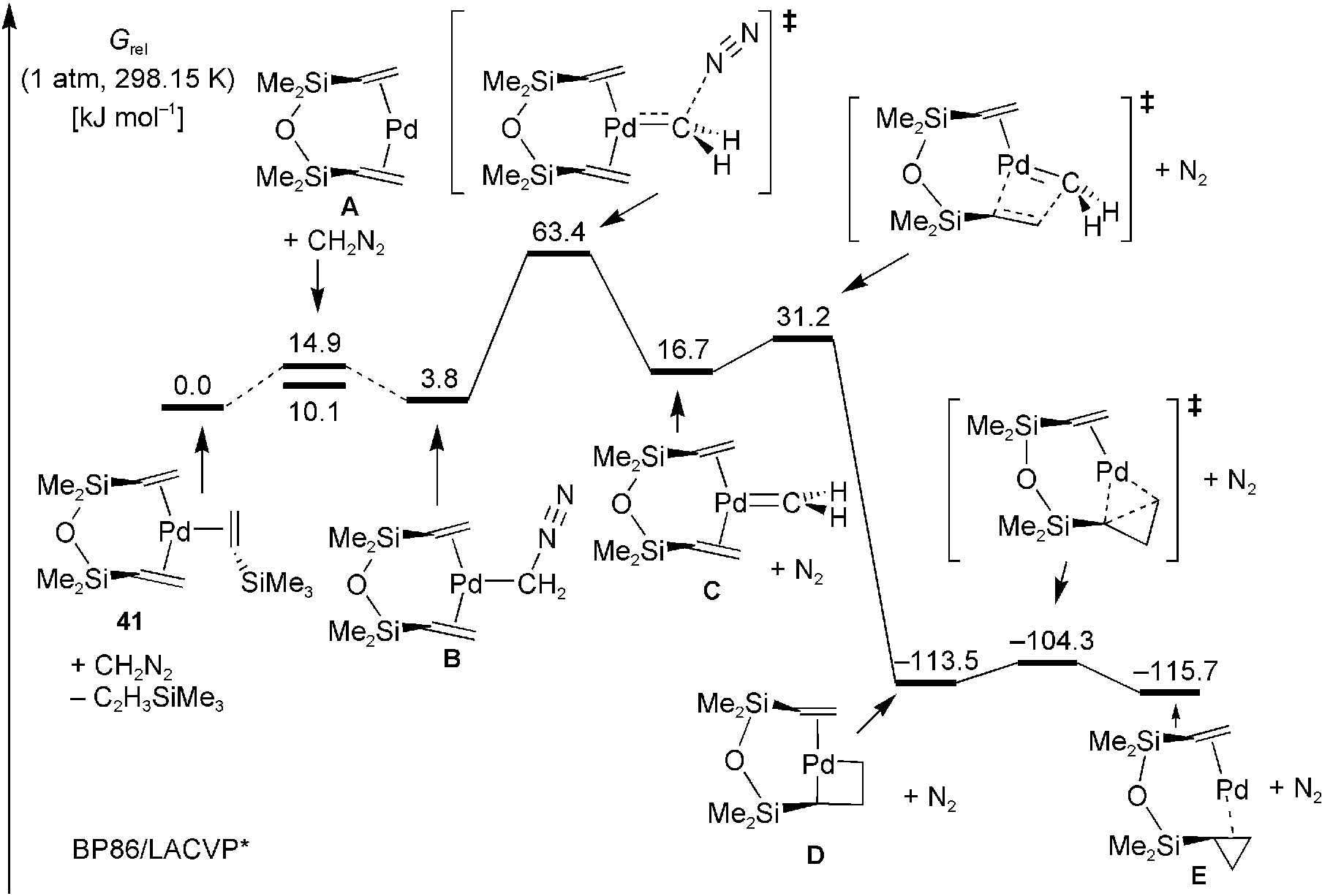
Quantum chemically computed Gibbs free-energy pathway for the cyclopropanation of DVTMS by diazomethane.
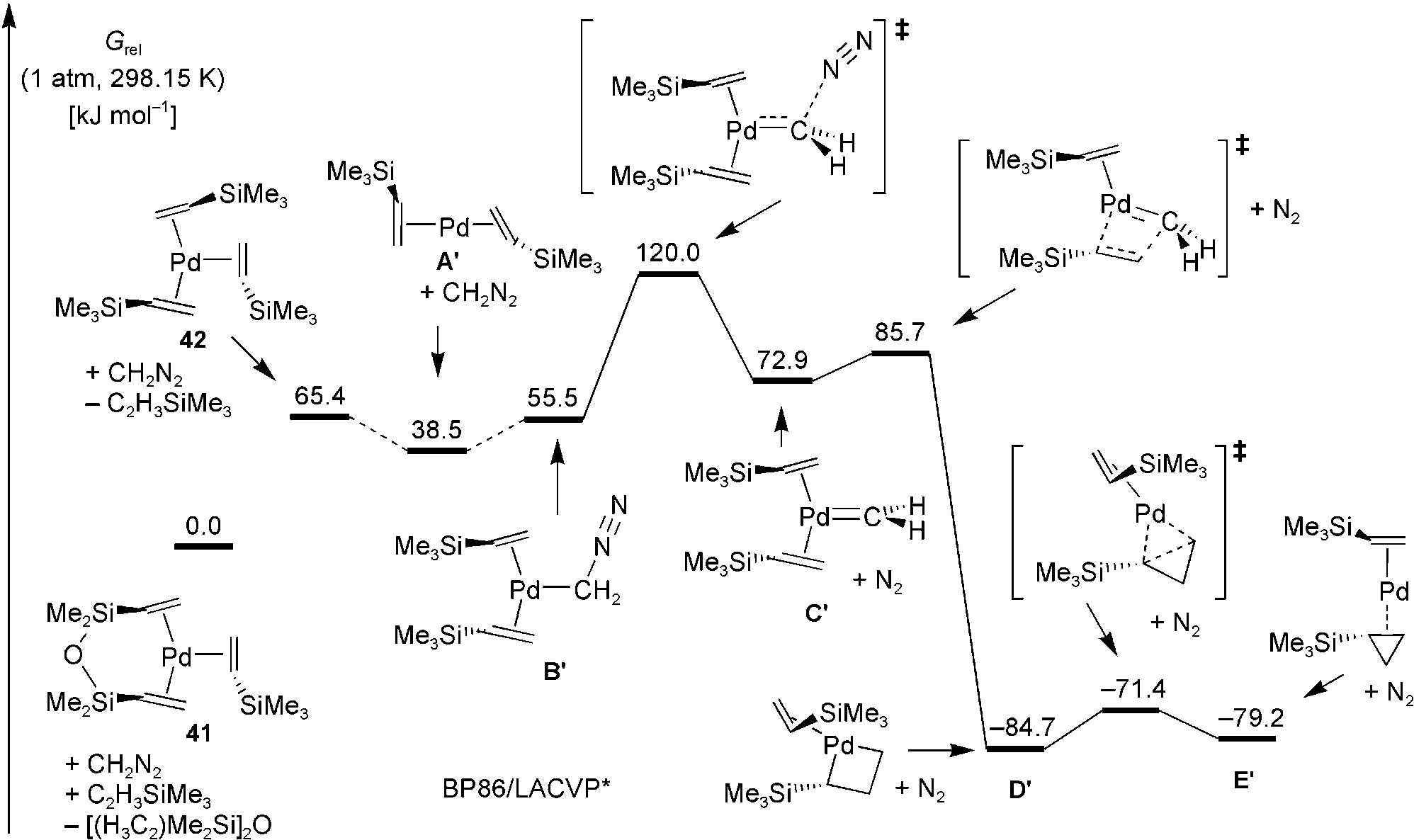
Quantum chemically computed lowest Gibbs free-energy pathway for the palladium(0)-catalyzed cy-clopropanation of trimethylvinylsilane by diazomethane.
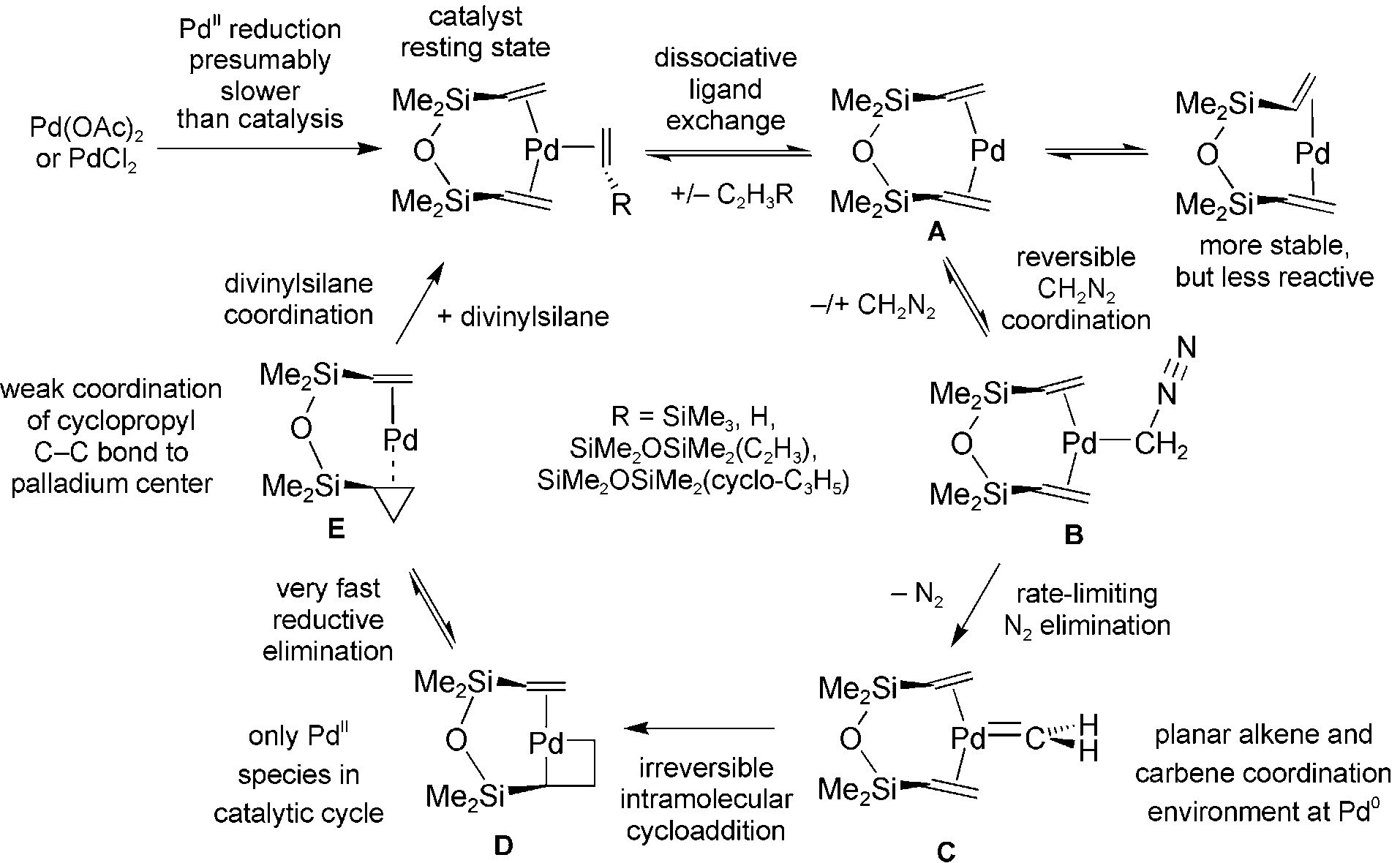
Cycle for the palladium-catalyzed cyclopropanation of DVTMS by diazomethane according to experimental observations and DFT calculations.
CAS number: 33419-42-0
Etoposide is a beta-D-glucoside, a furonaphthodioxole and an organic heterotetracyclic compound. It has a role as an antineoplastic agent and a DNA synthesis inhibitor. It is functionally related to a podophyllotoxin and a 4'-demethylepipodophyllotoxin.
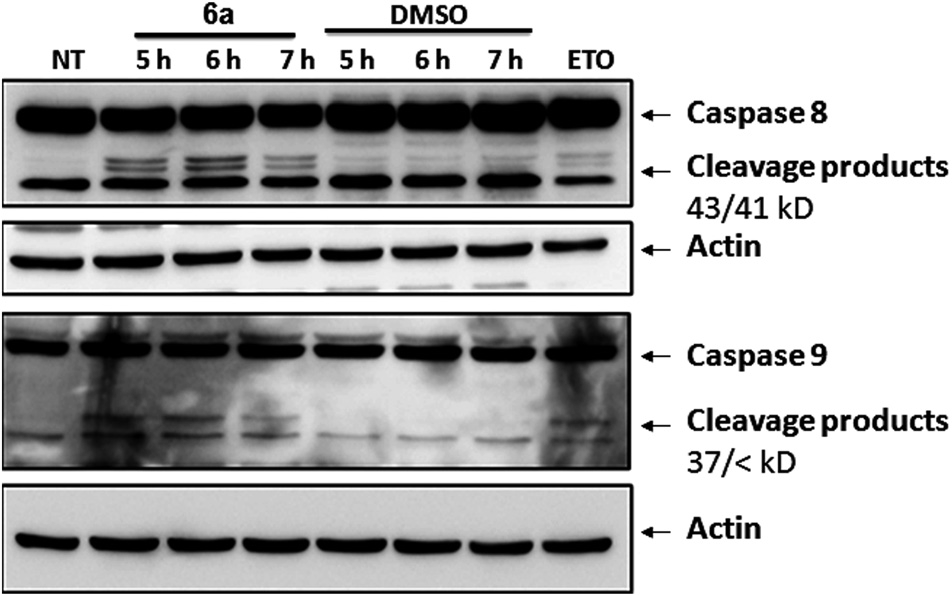
Study of early signaling events of apoptosis induced by compound 6a in Jurkat cells. Cells treated with Etoposide were included as positive control.
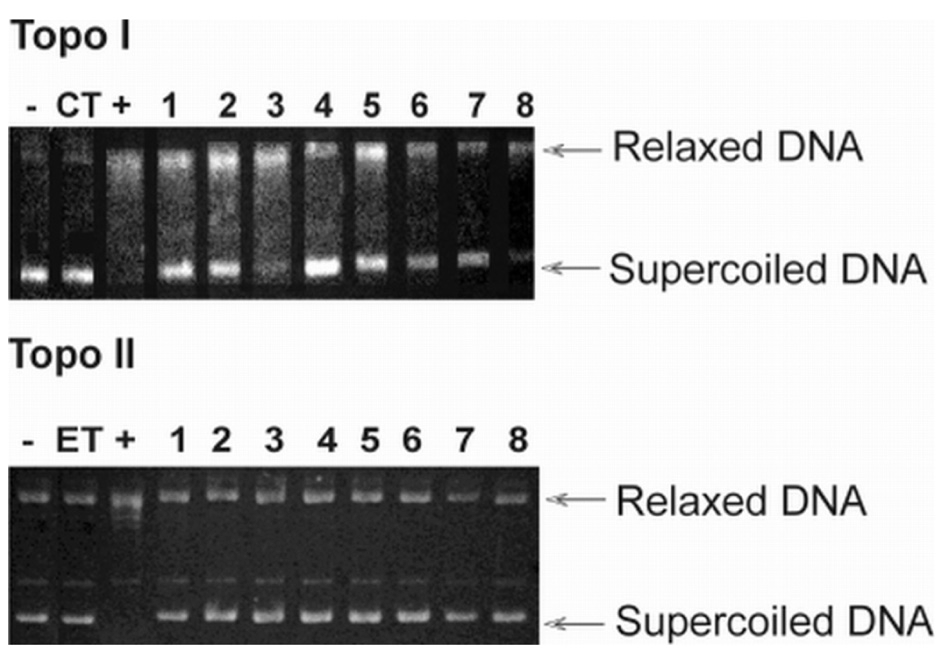
Inhibition of topoisomerase by compounds 1-8. Native pBR322 plasmid DNA (-lane) was incubated with 4 units of topoisomerase I (Topo I) and II (Topo II) without (+lane), with control (CT-camptothecin or ET-etoposide), or with drug (lanes 1-8). DNA was analyzed by 1% agarose gel electrophoresis. The gel was stained with ethidium bromide and photographed under UV light.