Chemicals list & Research Gallery
CAS number: 31096-91-0
N-Phenylindole, also known as 1-Phenyl-1H-indole, is an aromatic organic compound composed of an indole core with a phenyl group attached at the nitrogen atom (N1 position).
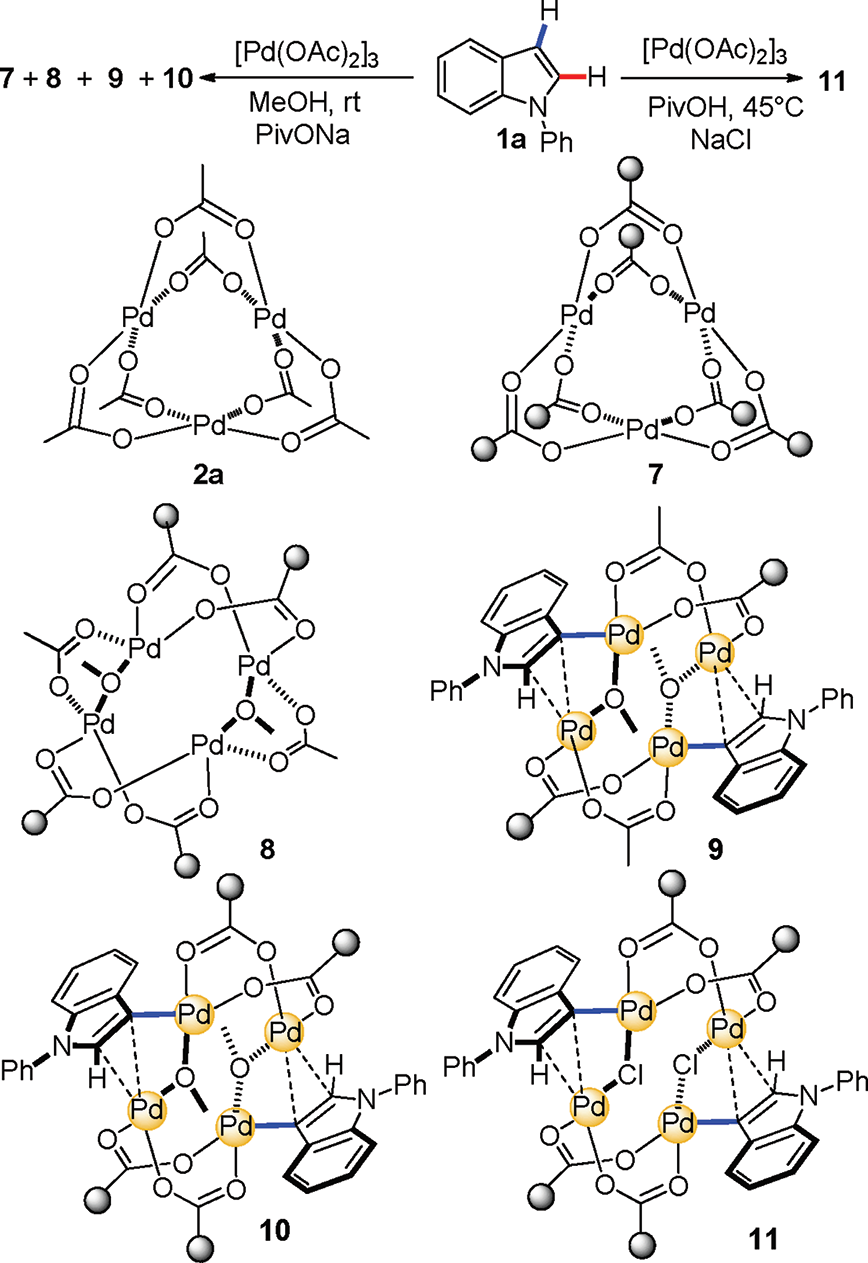
Regioselective Electrophilic Palladation at the C3 Position of N-Phenylindole
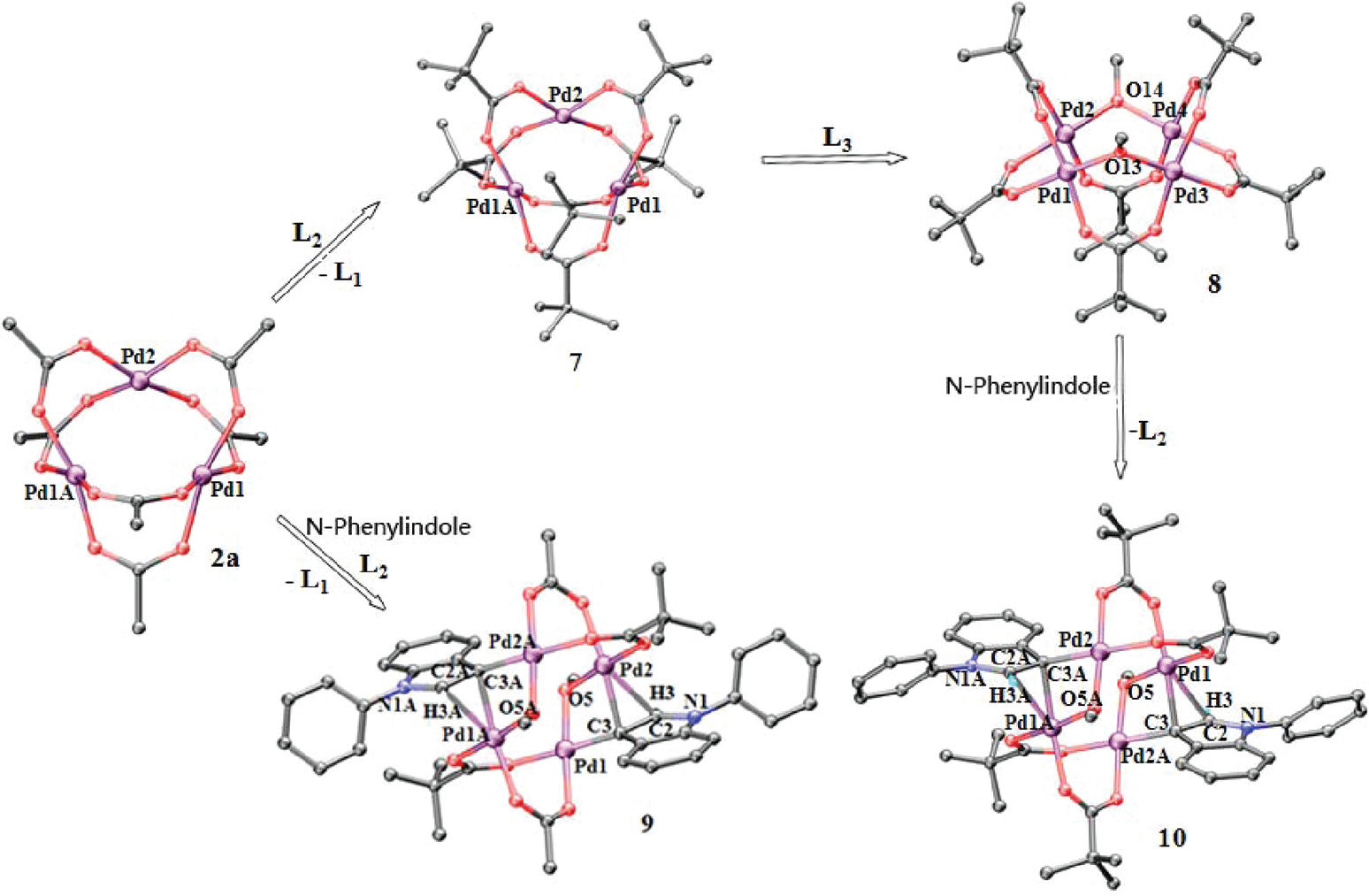
Crystal structures of 7−10 and the possible pathway to 10 through C3 palladation of N-phenylindole (L1 = OAc, L2 = PivO, L3 = MeO−).
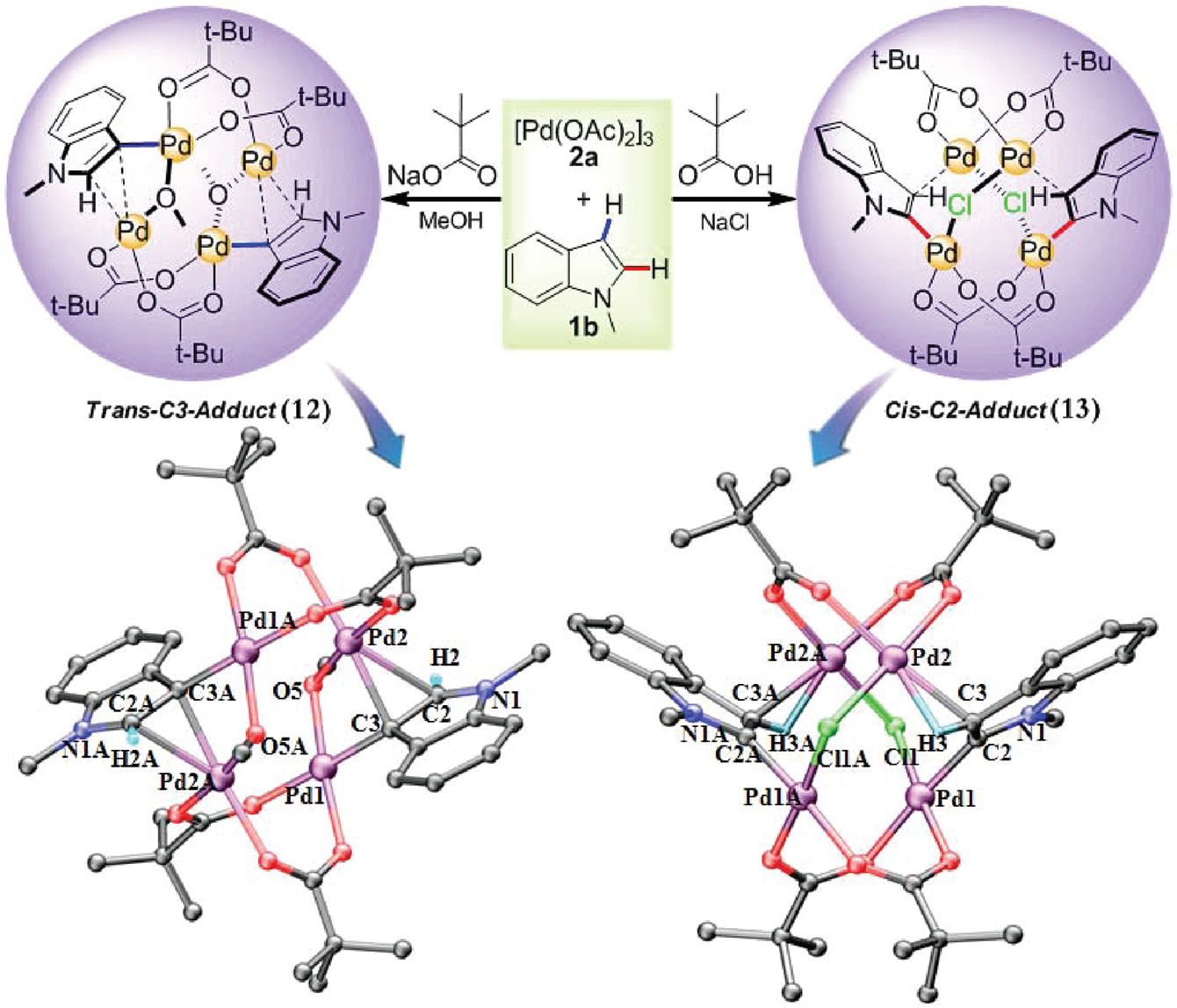
pH-Dependent Highly Regioselective Electrophilic Palladation at the C2 or C3 Position of N-Methylindole and X-ray Crystal Structures of Compounds 12 and 13
CAS number: 31135-62-3
An antimalarial drug used to treat susceptible infections with P. vivax, P. malariae, P. ovale, and P. falciparum.
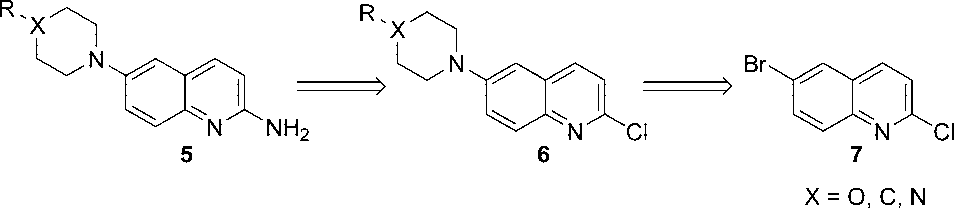
Retrosynthesis of 6-Substituted 2-Aminoquinolines
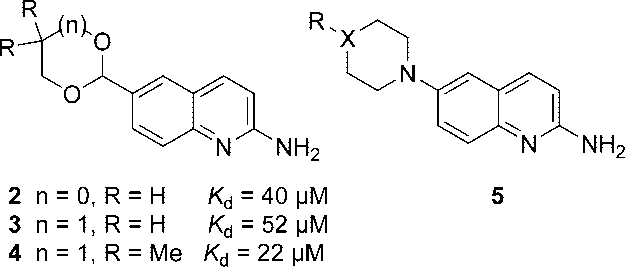
Equilibrium binding dissociation constants (Kd) of some 2-aminoquinolines reported previously.
CAS number: 31139-16-9
Cyanoboron, or more precisely sodium cyanoborohydride (NaCNBH3), is a chemical compound primarily used as a reducing agent in organic synthesis. It's known for being a milder reducing agent than sodium borohydride (NaBH4) and is particularly useful for selectively reducing imines to amines in the presence of other carbonyl compounds like ketones and aldehydes.
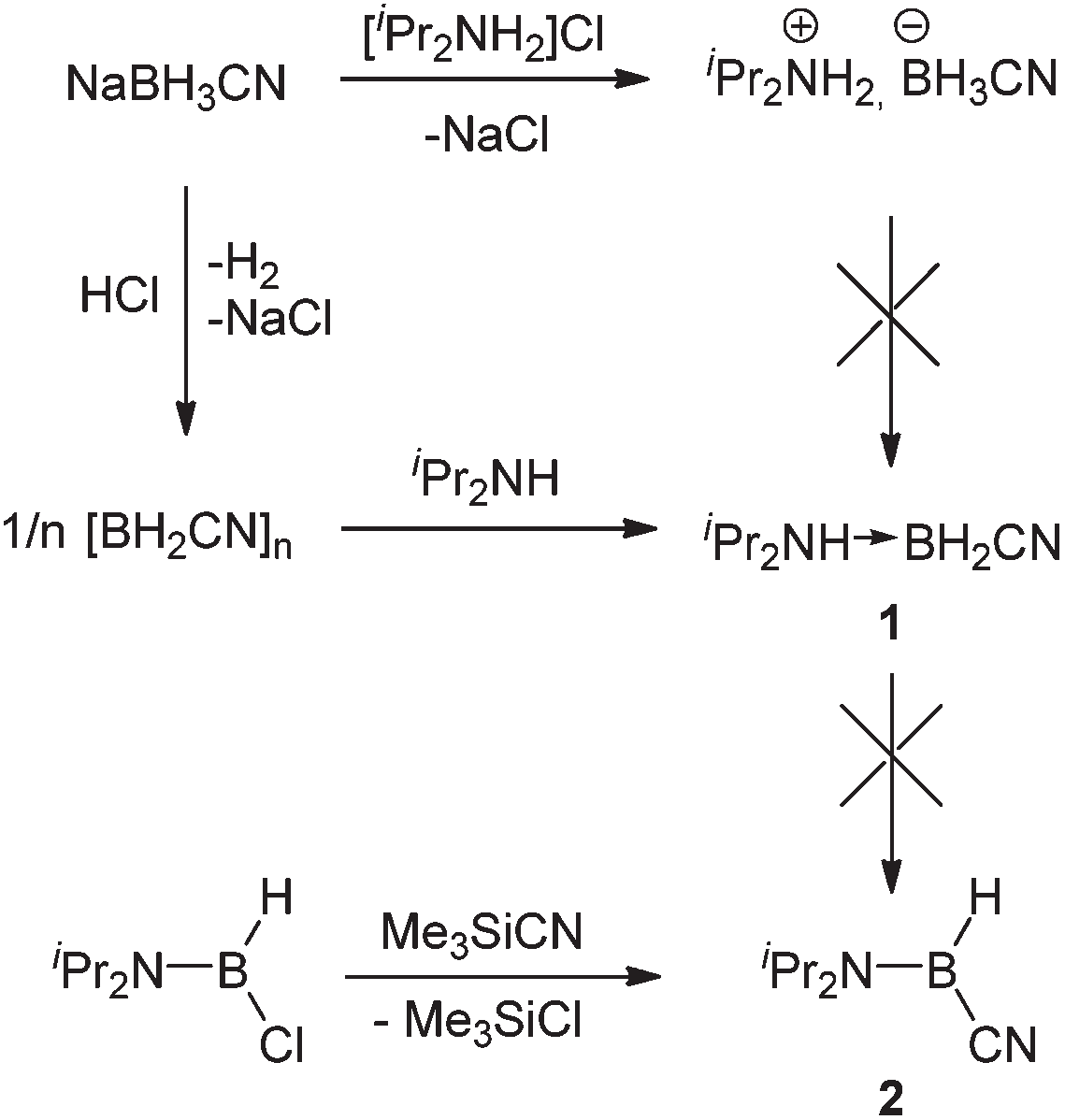
Synthesis of diisopropylamine-cyanoborane 1 and diisopropylamino-(cyano)borane 2.
CAS number: 31431-39-7
Mebendazole is a carbamate ester that is methyl 1H-benzimidazol-2-ylcarbamate substituted by a benzoyl group at position 5. It has a role as an antinematodal drug, a tubulin modulator and a microtubule-destabilising agent. It is a member of benzimidazoles, a carbamate ester and an aromatic ketone. It derives from a hydride of a 1H-benzimidazole.
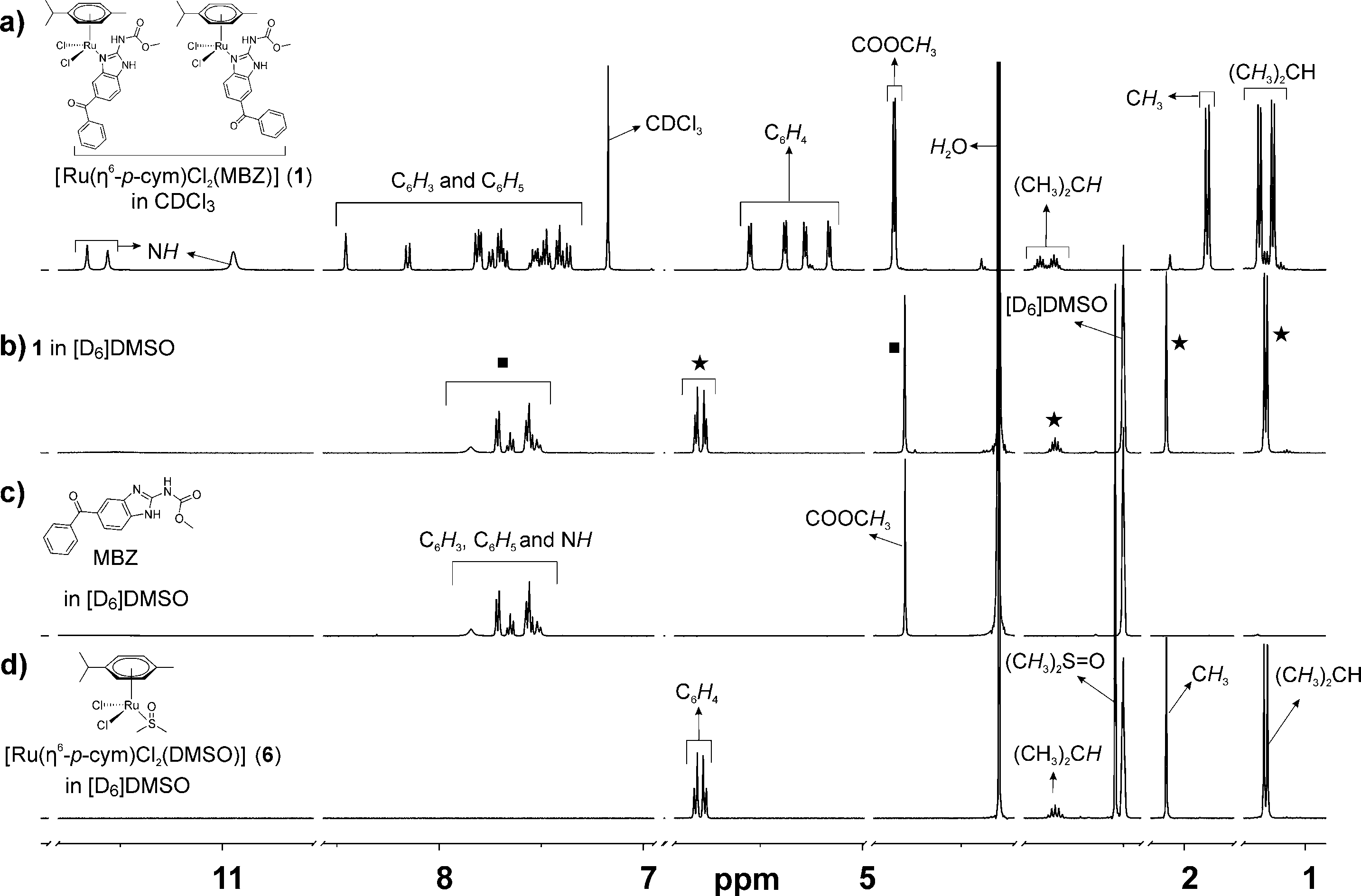
Complete dissociation of mebendazole (MBZ) ligand from the original framework of complex 1 in DMSO.
CAS number: 3144-16-9
(S)-camphorsulfonic acid is the S enantiomer of camphorsulfonic acid. It is a conjugate acid of a (S)-camphorsulfonate. It is an enantiomer of a (R)-camphorsulfonic acid.
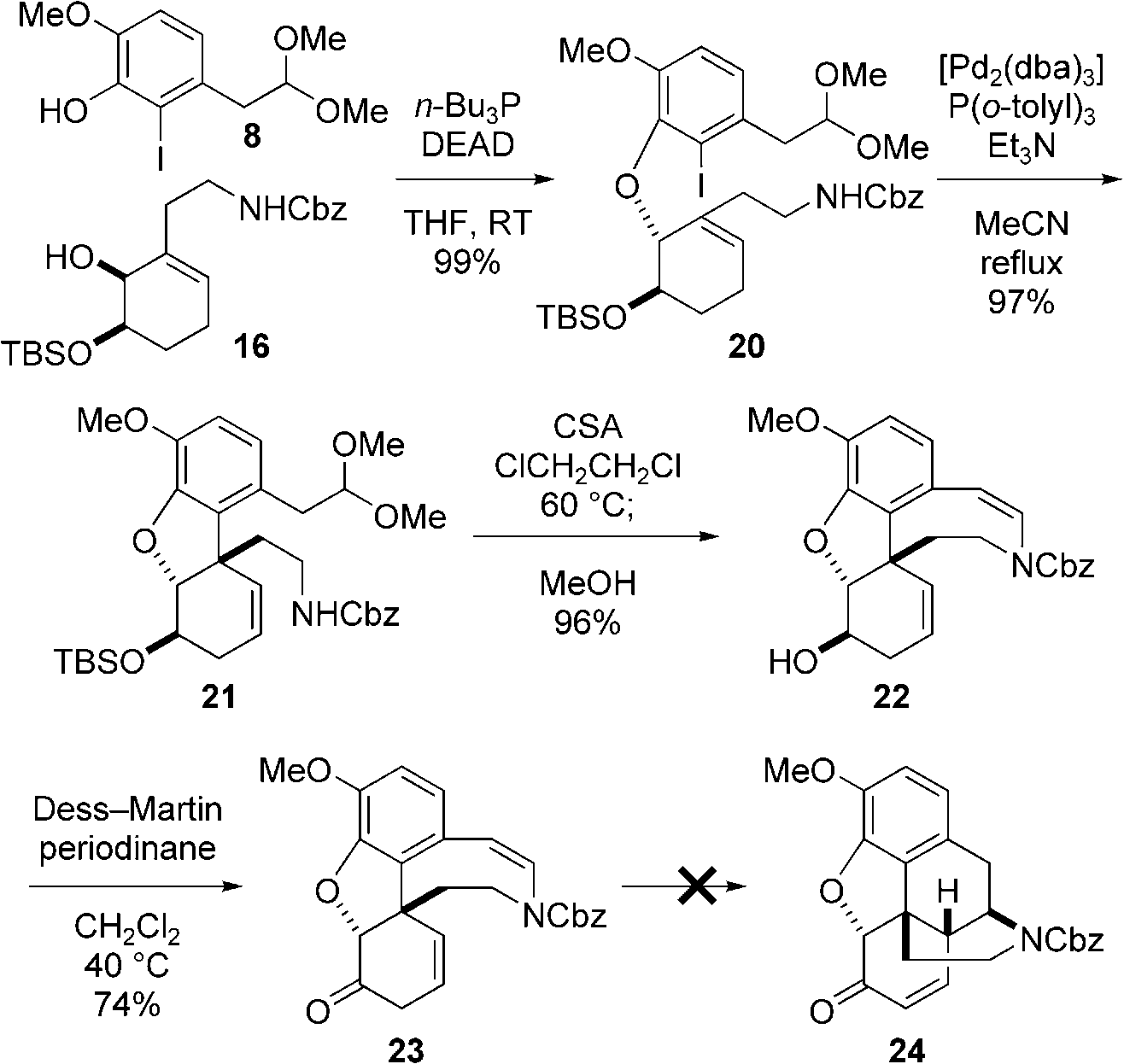
Attempted Mannich-type reaction. DEAD=diethyl azodicarboxylate, dba=(E,E)-dibenzylideneacetone, CSA=camphorsulfonic acid.
CAS number: 3170-80-7
Trichloromethane is an organic chemical compound also known as chloroform. It is a volatile, colorless liquid with a sweet, ether-like odor. While historically used as an anesthetic, its main uses today are as a solvent, a chemical intermediate in various industrial processes, and a precursor in the production of refrigerants and PTFE.
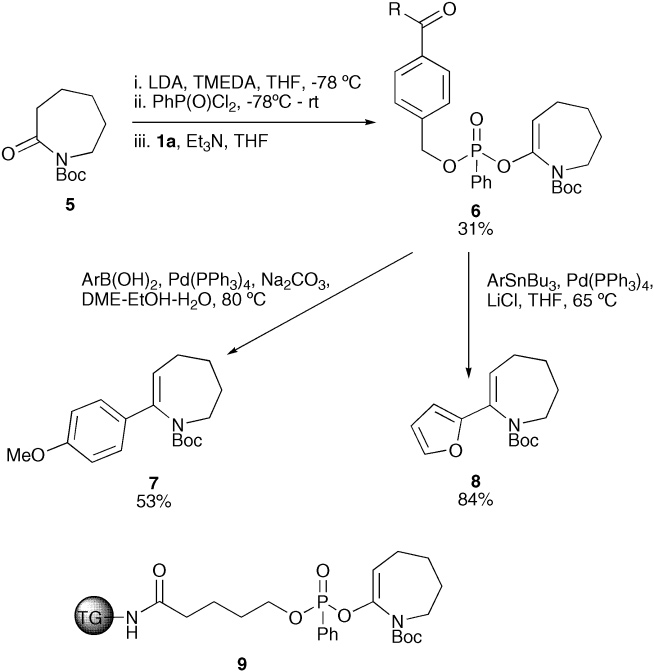
Although phosphonate 6 was acid labile and decomposed overnight in chloroform, it still underwent efficient cross-coupling reactions.
CAS number: 319460-85-0
Axitinib is a Kinase Inhibitor. The mechanism of action of axitinib is as a Receptor Tyrosine Kinase Inhibitor.
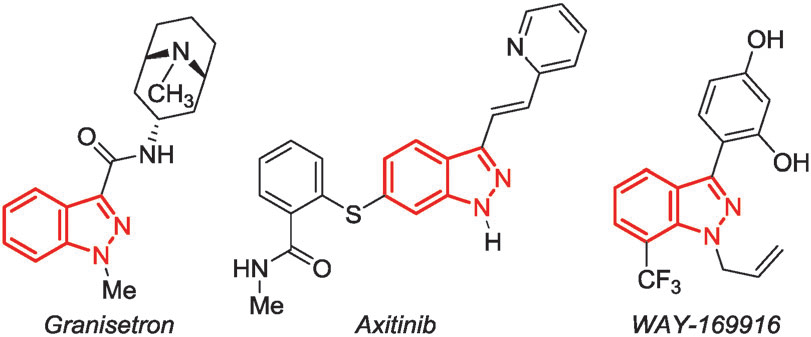
Some drugs and drug candidates containing the 1H-indazole structure.
CAS number: 3198-30-9
Terephthalate(2-) is a dicarboxylic acid dianion obtained by the deprotonation of the carboxy groups of terephthalic acid. It is a conjugate base of a terephthalate(1-).
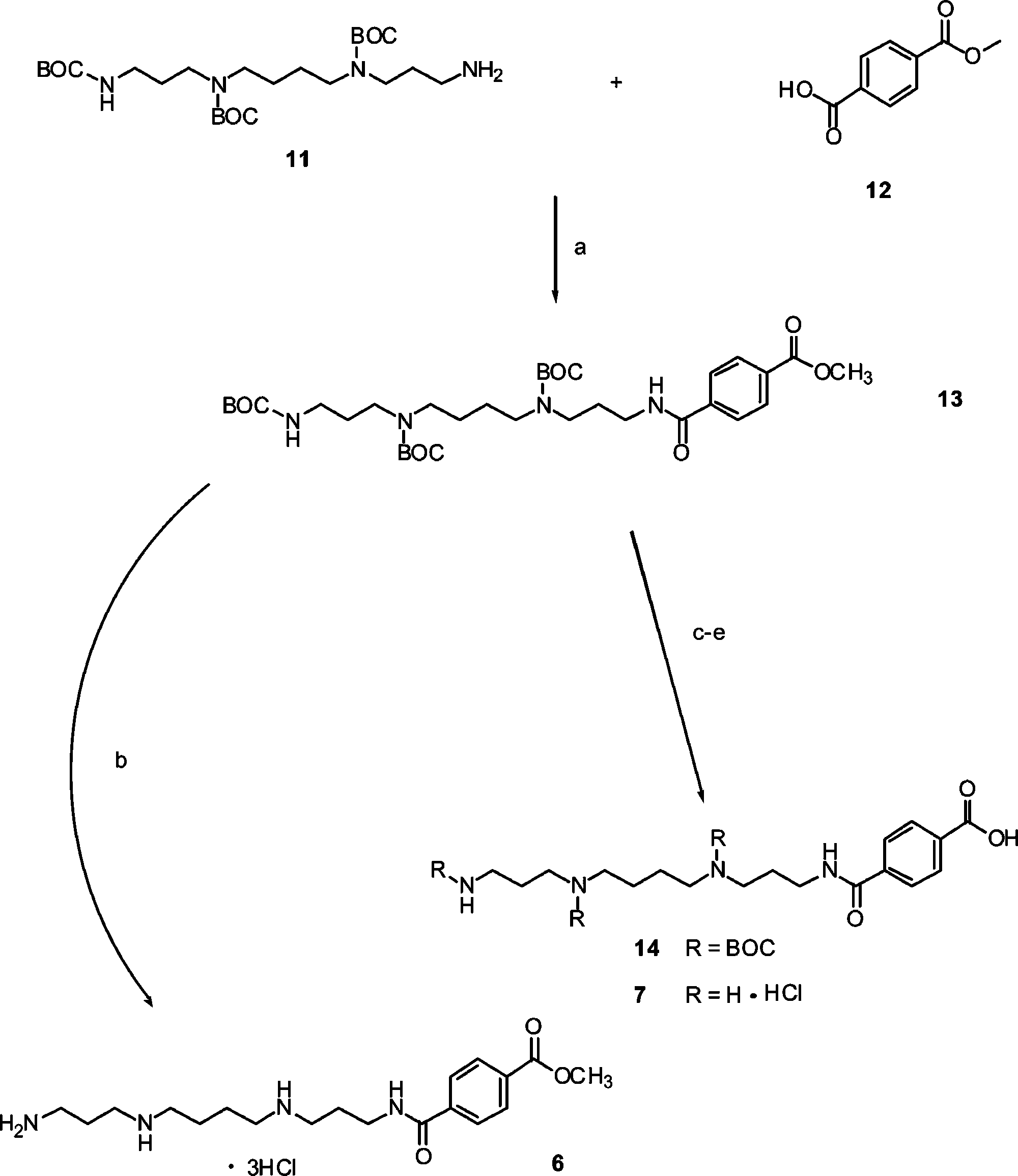
Synthesis of SPD-terephthalates 6 and 7
CAS number: 32222-06-3
Calcitriol is a medication that treats low calcium levels caused by kidney disease. It can also treat parathyroid gland conditions.
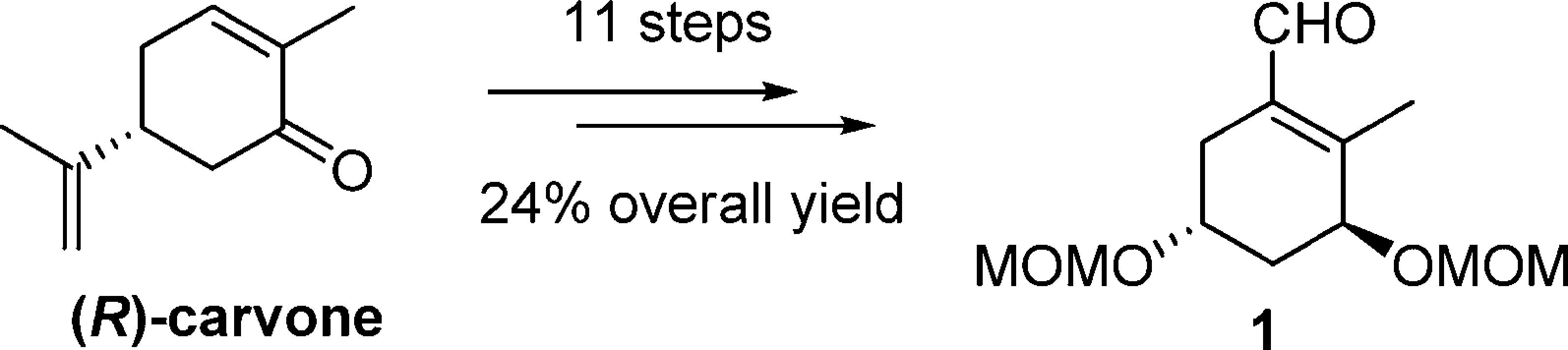
Enantioselective synthesis of a key A-ring intermediate for the preparation of 1α,25-dihydroxyvitamin D3
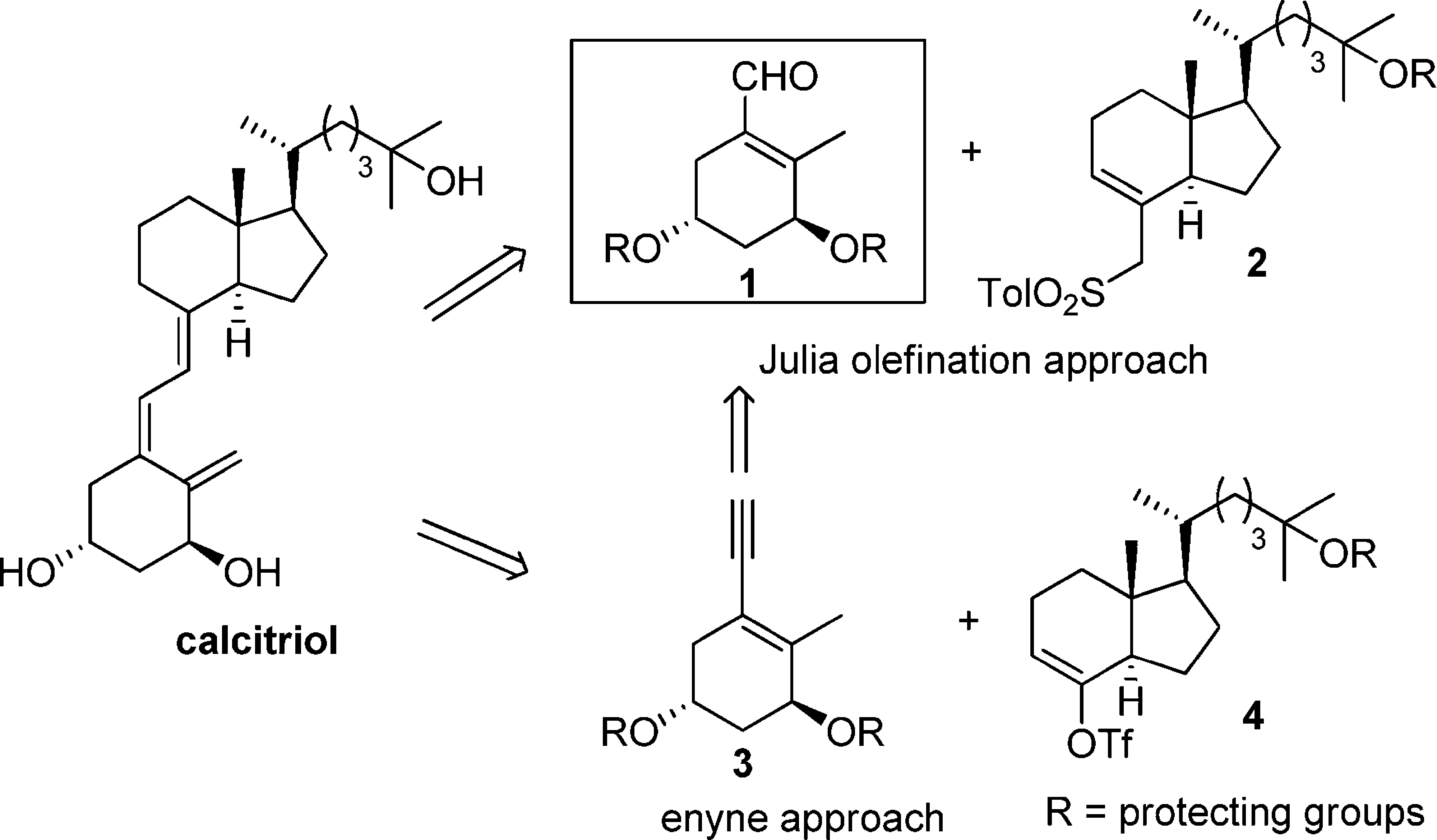
α,β-Unsaturated aldehyde 1 serves as a key intermediate in the synthesis of calcitriol.
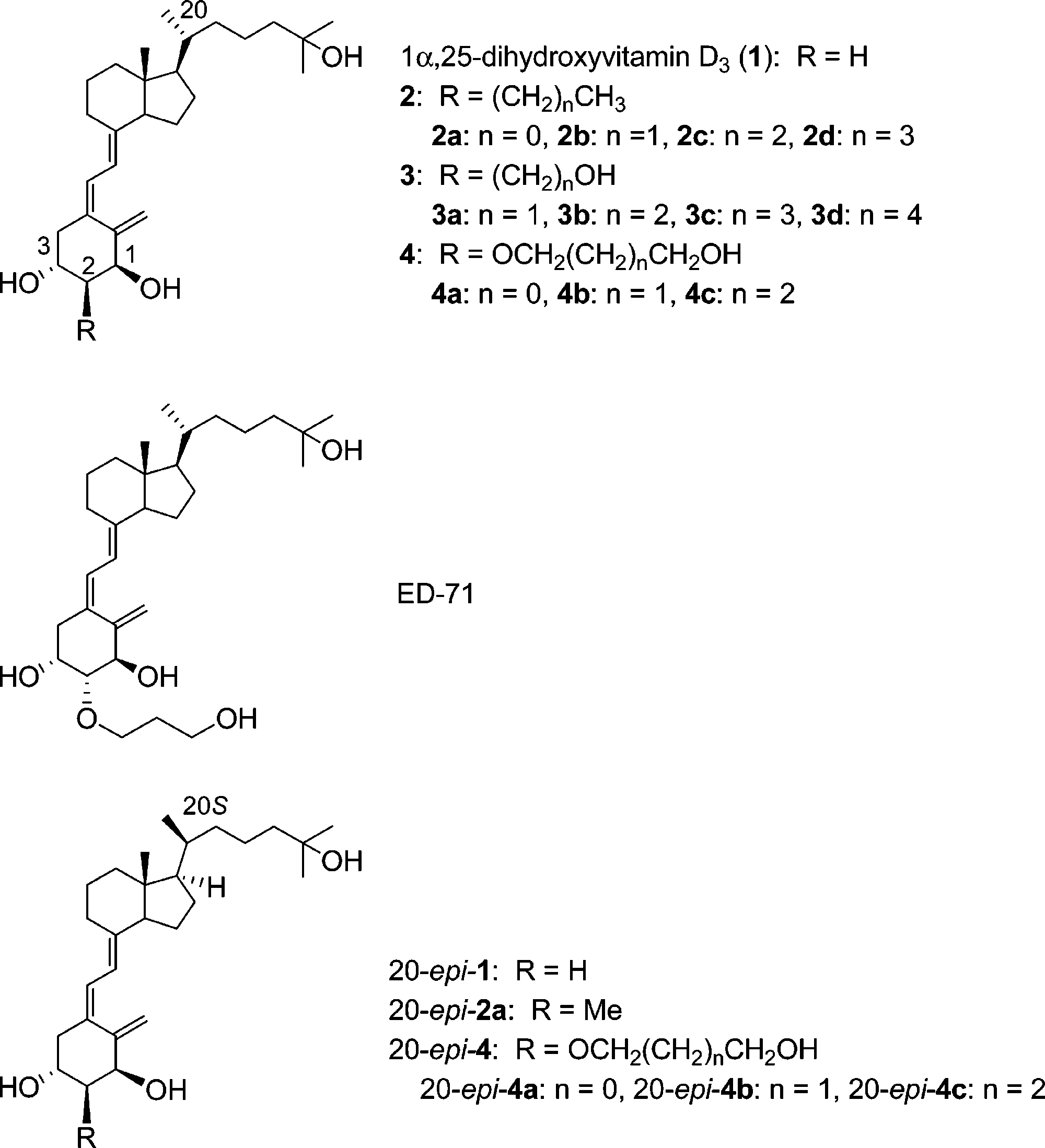
Structures of 1α,25-dihydroxyvitamin D3 (1) (and its 2R-substituted analogues 2-4), ED-71, and 20-epi-1 (and its 2R-substituted analogues 20-epi-2a and 20-epi-4a -c).
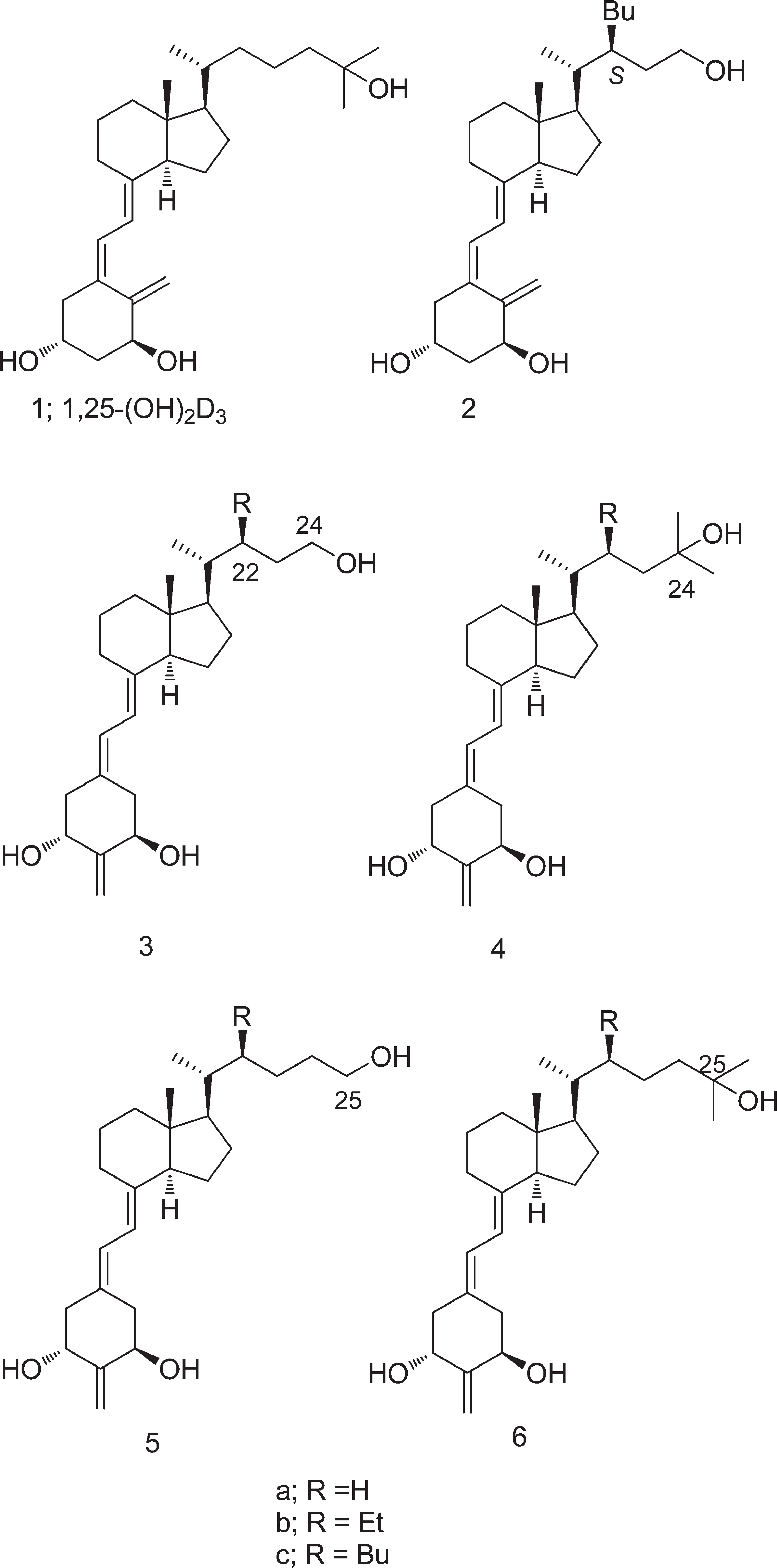
Structures of 1,25-(OH)2D3 (1) and Vitamin D Analogues
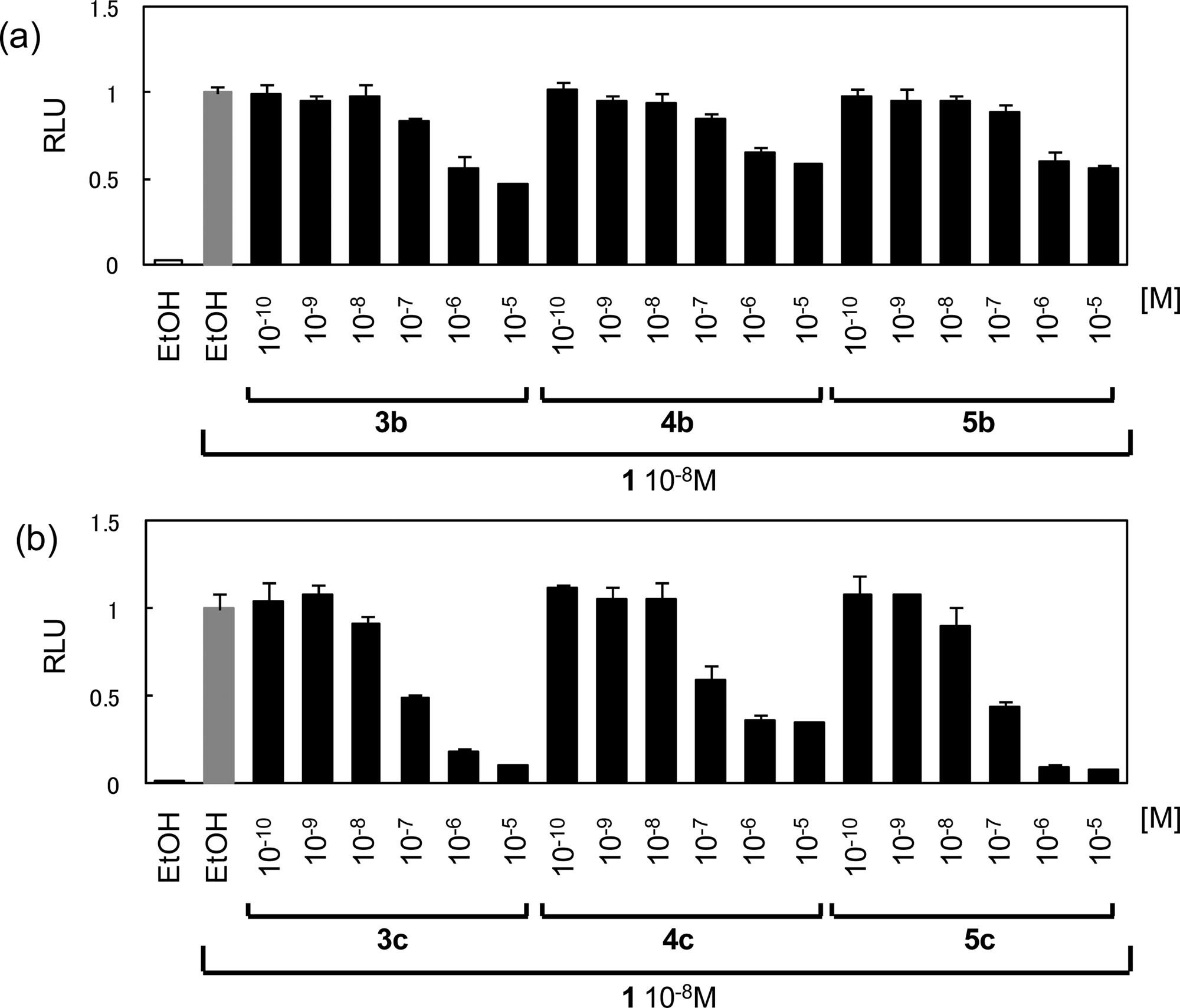
Inhibitory effect on the transactivation induced by 1,25-(OH)2D3 (1) was evaluated.
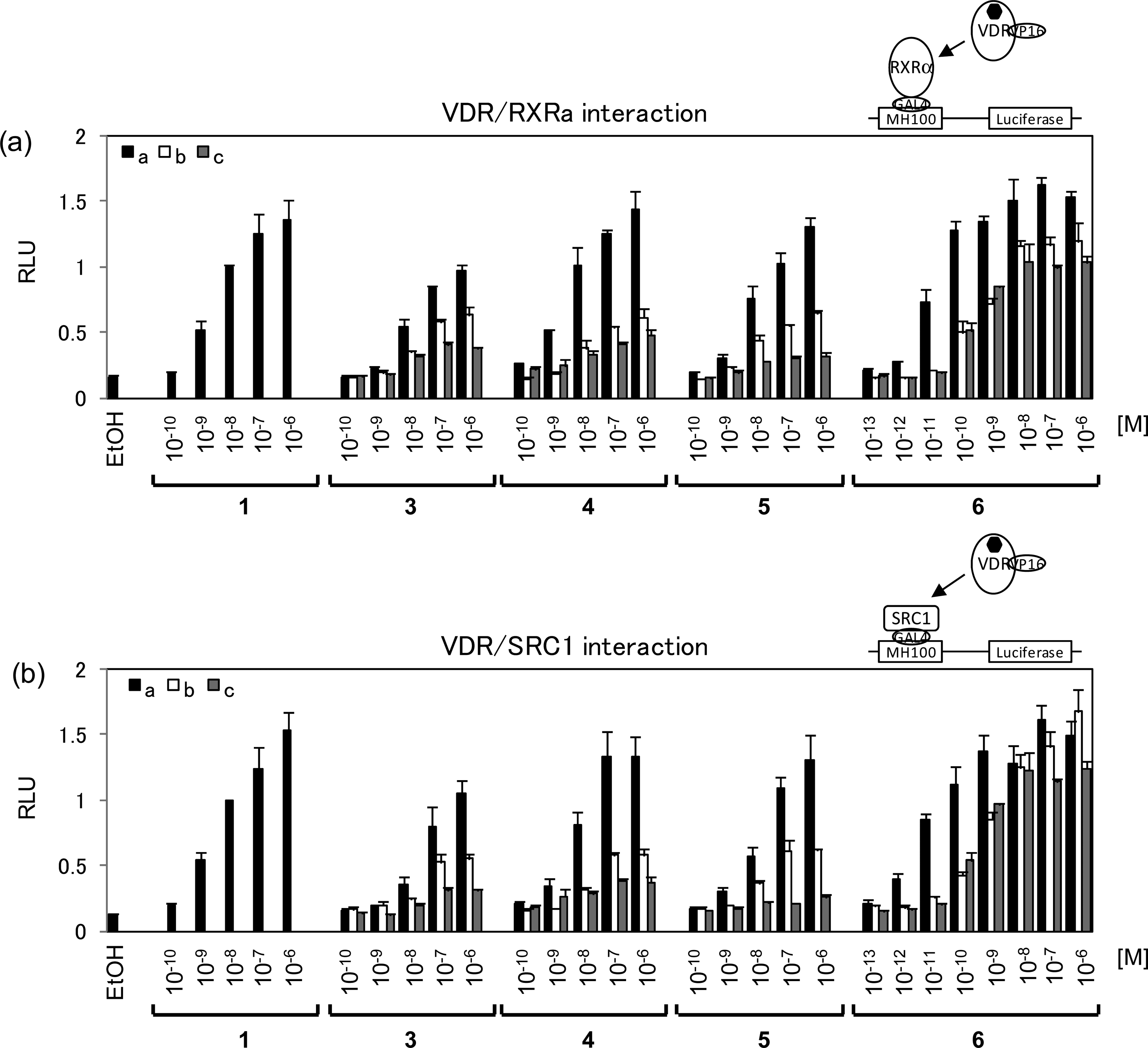
RXRR and SRC-1 recruitment to VDR by 1,25-(OH)2D3 (1) and synthetic compounds (3a-c, 4a-c, 5a-c, and 6a-c) in Cos7 cells.
CAS number: 32264-87-2
2-Cyclobutene-1-one is a cyclic ketone. It features a four-membered ring (cyclobutene) with a ketone group (C=O) attached to one of the carbons.
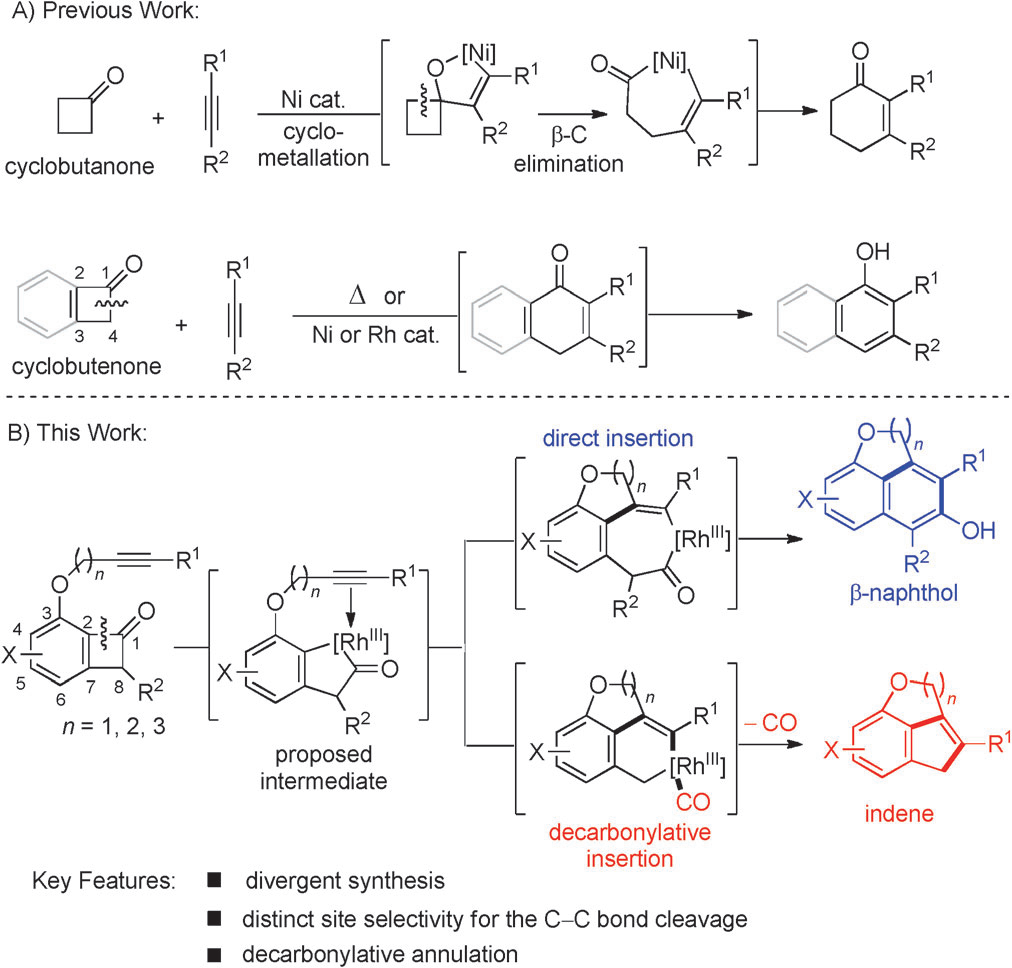
C C bond cleavage in cyclobutenones and cyclobutanones.