Ethyl cyanoacetate
CAS number: 105-56-6
Ethyl cyanoacetate appears as a colorless liquid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Related images
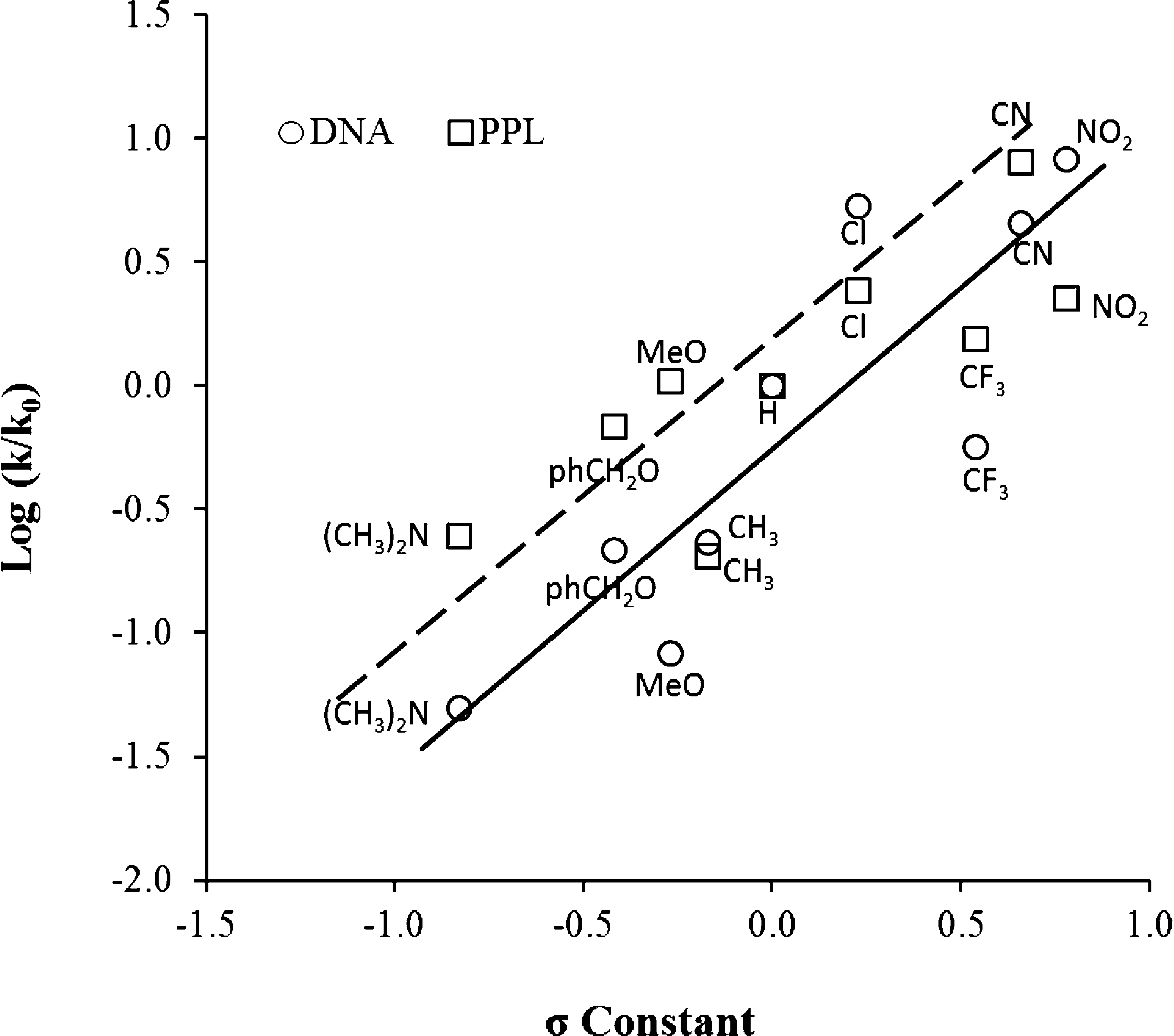
Hammett plots of the Knoevenagel condensation reaction of para-substituted benzaldehydes with ethyl cyanoacetate catalyzed by sodium salt of DNA fragments (circles) and PPL (squares).
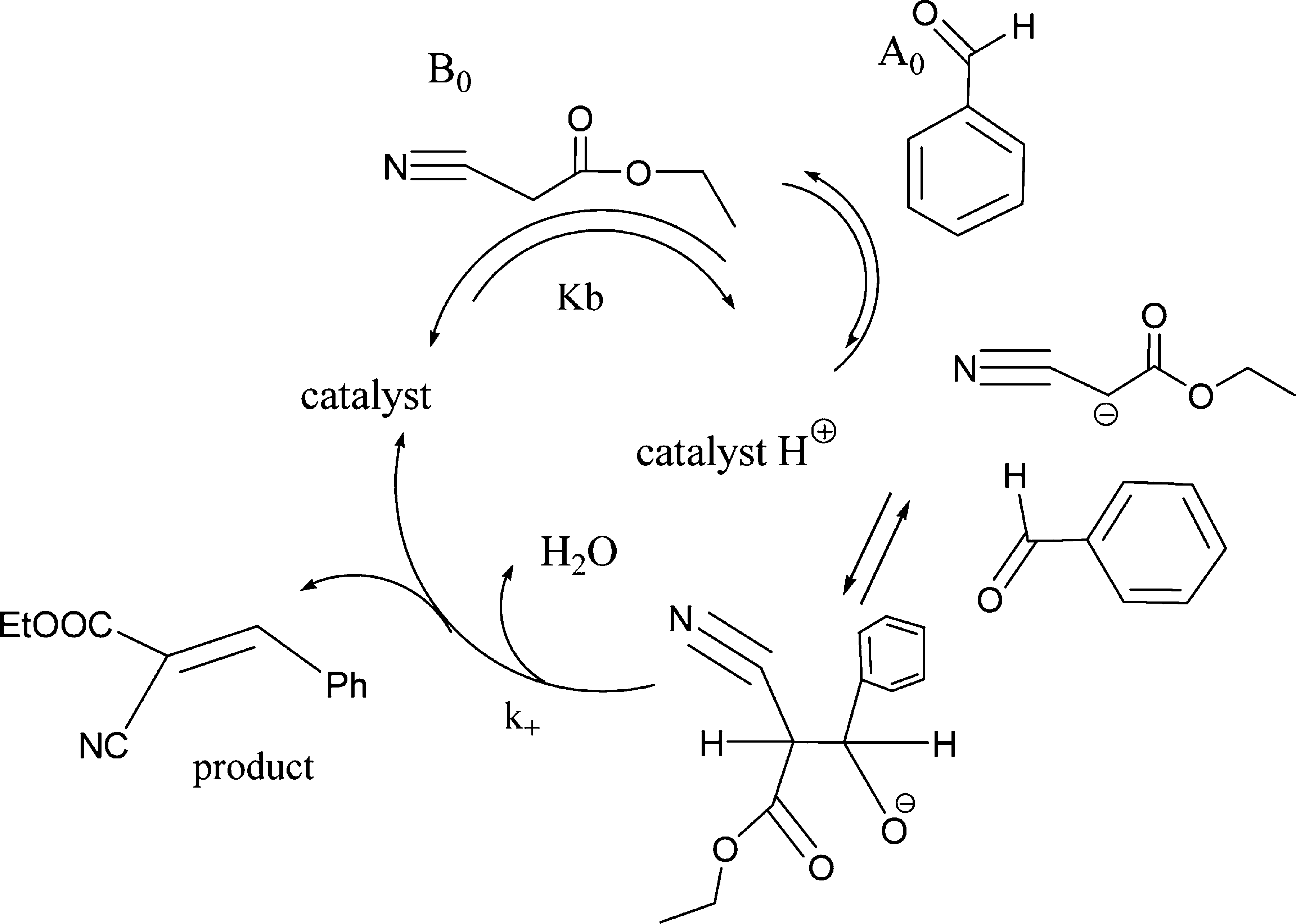
Proposed Kinetic Mechanism DNA/RNA-Salts Appears To Progress via the Shown Catalytic Cycle
Related Questions and Answers
Q: What reaction were benzaldehyde and ethyl cyanoacetate used in to evaluate catalytic performance?
A: Benzaldehyde and ethyl cyanoacetate were used in the Knoevenagel condensation reaction to evaluate the catalytic performance of metal-doped ETS-10 zeolites, with ethyl α-cyanocinnamate (ECC) as the target product.