Tetrabutylammonium
CAS number: 10549-76-5
Tetrabutylammonium is a chemical compound characterized by a positively charged nitrogen atom bonded to four butyl groups (C4H9), forming a quaternary ammonium cation. It's often used in the form of salts with various anions, such as bromide, chloride, fluoride, or perchlorate.
Related images
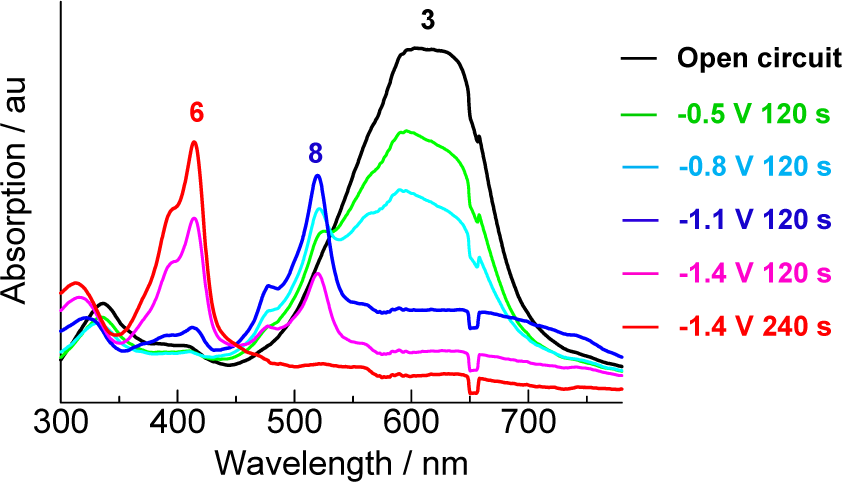
Electrochromic behavior of 3 in THF with tetrabutylammonium perchlorate as the supporting electrolyte at 0.0, −0.5, −0.8, −1.1, −1.4 (V vs Ag/Ag+ as a reference electrode). Platinum was used as the working and counter electrodes.
Related Questions and Answers
No related questions yet