Chemicals list & Research Gallery
CAS number: 4619-74-3
2,4,6-Tribromo-m-cresol, also known as tribromometacresol, is an organic chemical compound. It's a brominated derivative of m-cresol (3-methylphenol) where three hydrogen atoms on the benzene ring have been replaced by bromine atoms. It has been used as an antifungal agent.
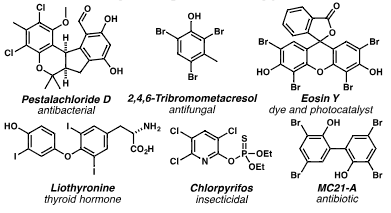
Selected haloarenes: Pestalachloride D, 2,4,6-Tribromo-m-cresol, Eosine Y, Liothyronine, Chlorpyrifos, mC21-A.
CAS number: 462-80-6
Benzyne is a highly reactive organic molecule that has not been isolated. It's an extremely reactive intermediate, meaning it's not stable enough to be isolated but can be observed as a fleeting species during certain chemical reactions.
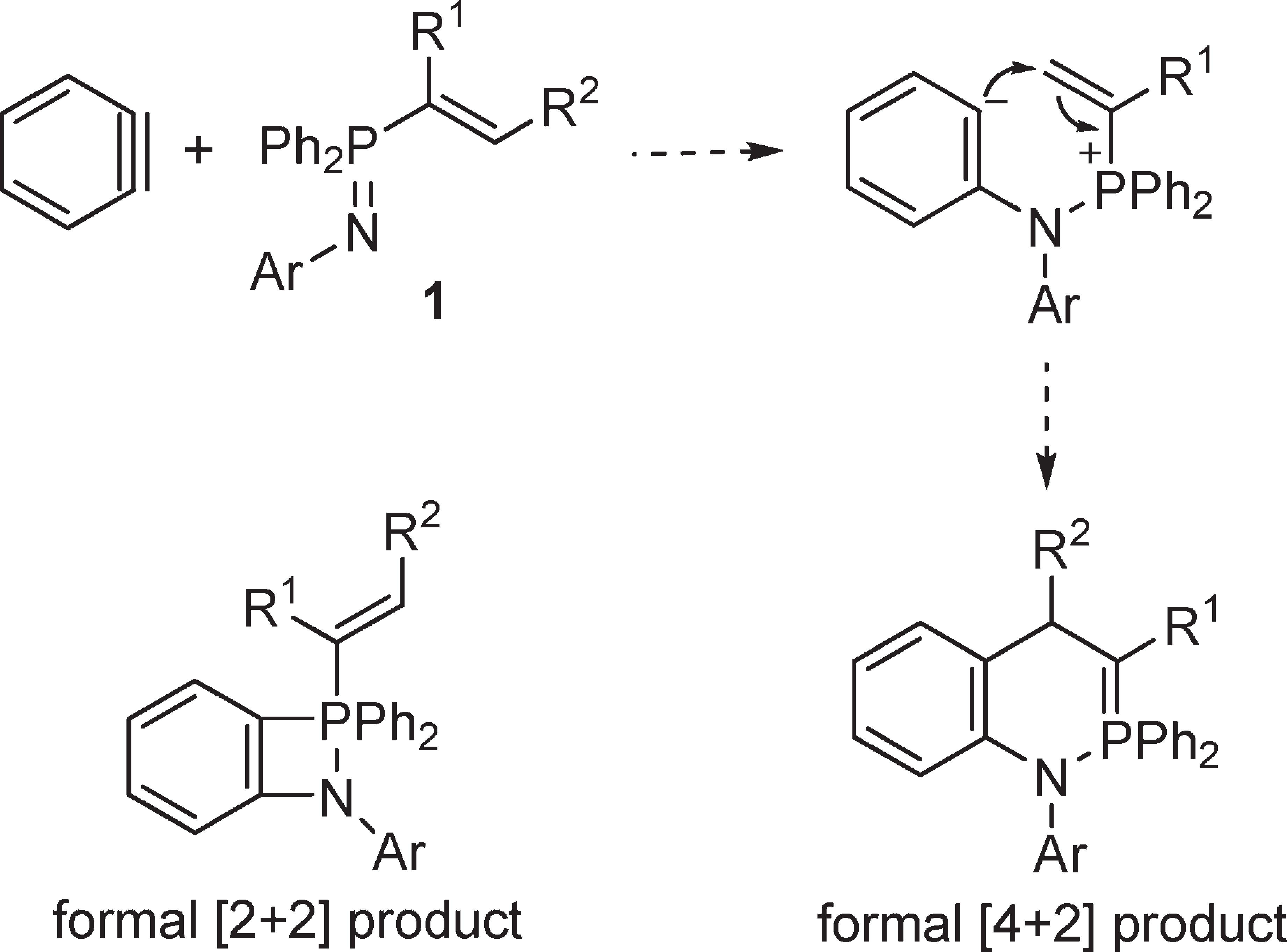
Possible Products of the Reaction of Benzyne and P-Alkenyl-λ5-phosphazenes
CAS number: 463-49-0
An allene is an organic molecule characterized by two adjacent carbon-carbon double bonds. It's also known as a cumulated diene.

Substrate scope of the orthoboration reaction. Reaction conditions: propadiene 1 (1 eq.), BnNH2 (5 mol%), PPh3 (10 mol%), B2pin2 (1.1 eq.), CuSO4 (1 mol%). a Using 1% TPGS-750-M. b Using 3:1 toluene:water as solvent.
CAS number: 465-29-2
Bornane-2,3-dione is a bornane monoterpenoid that is bicyclo[2.2.1]heptane substituted by methyl groups at positions 1, 7 and 7 and oxo groups at positions 2 and 3. It is a bornane monoterpenoid and a carbobicyclic compound.
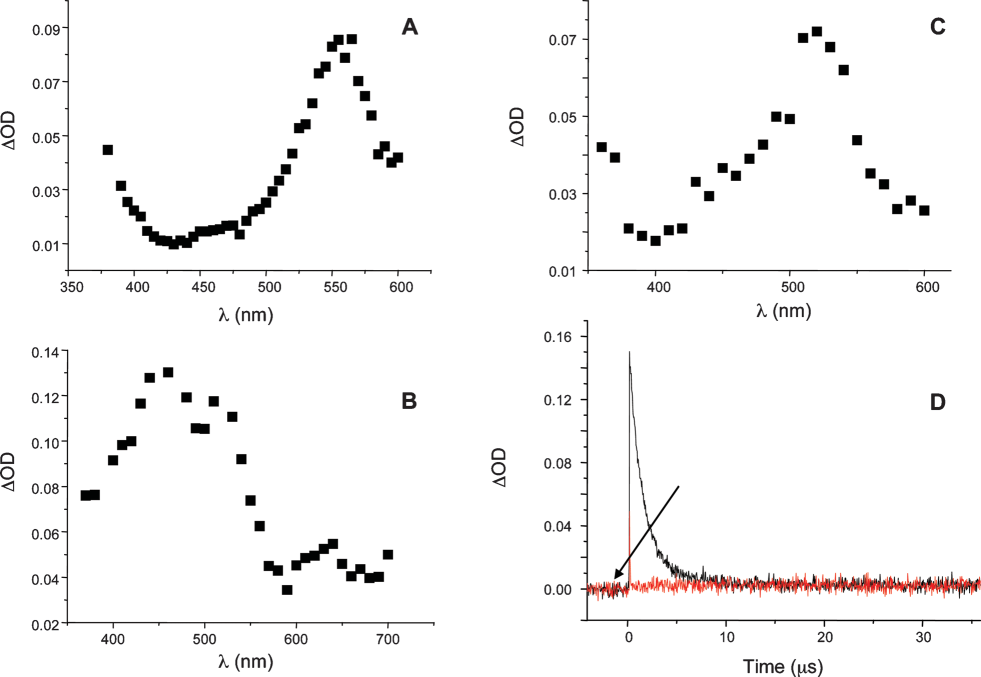
Transient absorption spectra of (A) 4, (B) 5, (C) 6 in acetonitrile at time t = 0 after laser excitation at 355 nm. (D) Transient decay at 460 nm upon addition of camphorquinone to 5 under argon.
CAS number: 466-97-7
Normorphine is a conjugate of morphine that is excreted renally, accounting for approximately 5% to 10% of the total excretion of morphine in the body.
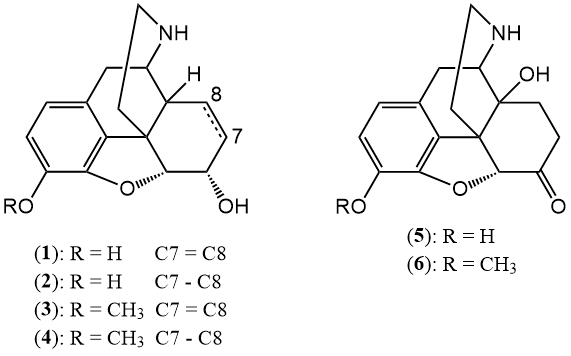
Normorphine derivatives.
CAS number: 468-10-0
Morphinan is a morphinane alkaloid and an isoquinoline alkaloid fundamental parent.
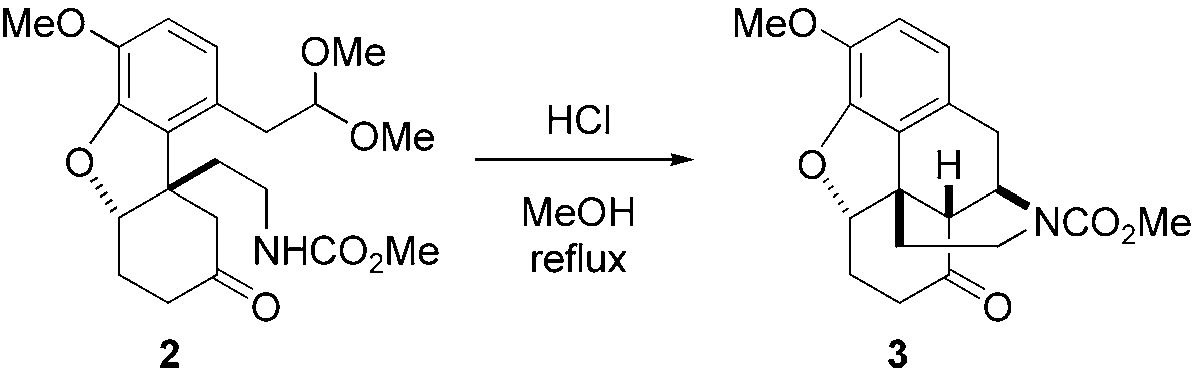
Mannich-type reaction to construct the morphinan skeleton.
CAS number: 469-22-7
Eseroline is a pyrroloindole that is 1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indole substituted by methyl groups at positions 1, 3a and 8 and a hydroxy group at position 5. It is a metabolite of physostigmine and causes neuronal cell death by a mechanism involving loss of cell ATP. It has a role as an opioid analgesic and a human xenobiotic metabolite. It is a member of phenols and a pyrroloindole.
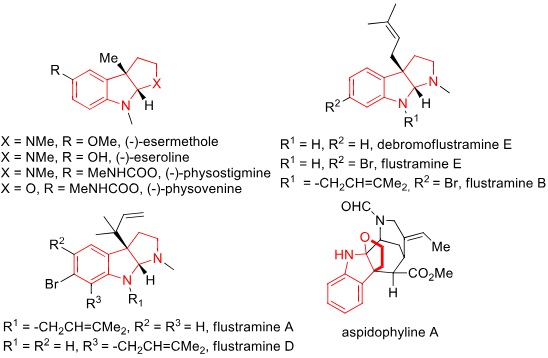
Indole Alkaloids Bearing FIH Skeletons: (-)-Esermethole, (-)-Eseroline, (-)-physostigmine, (-)-Physovenine, Flustramine E, Flustramine B, Flustramine A, Aspidophyline A.
CAS number: 471-25-0
Propynoic acid is a terminal acetylenic compound that is a 3-carbon, straight-chain, monounsaturated fatty acid having one acetylenic bond. It has a role as a xenobiotic metabolite. It is an acetylenic fatty acid, a monounsaturated fatty acid, a short-chain fatty acid, a terminal acetylenic compound and an alpha,beta-unsaturated monocarboxylic acid. It is a conjugate acid of a propynoate.
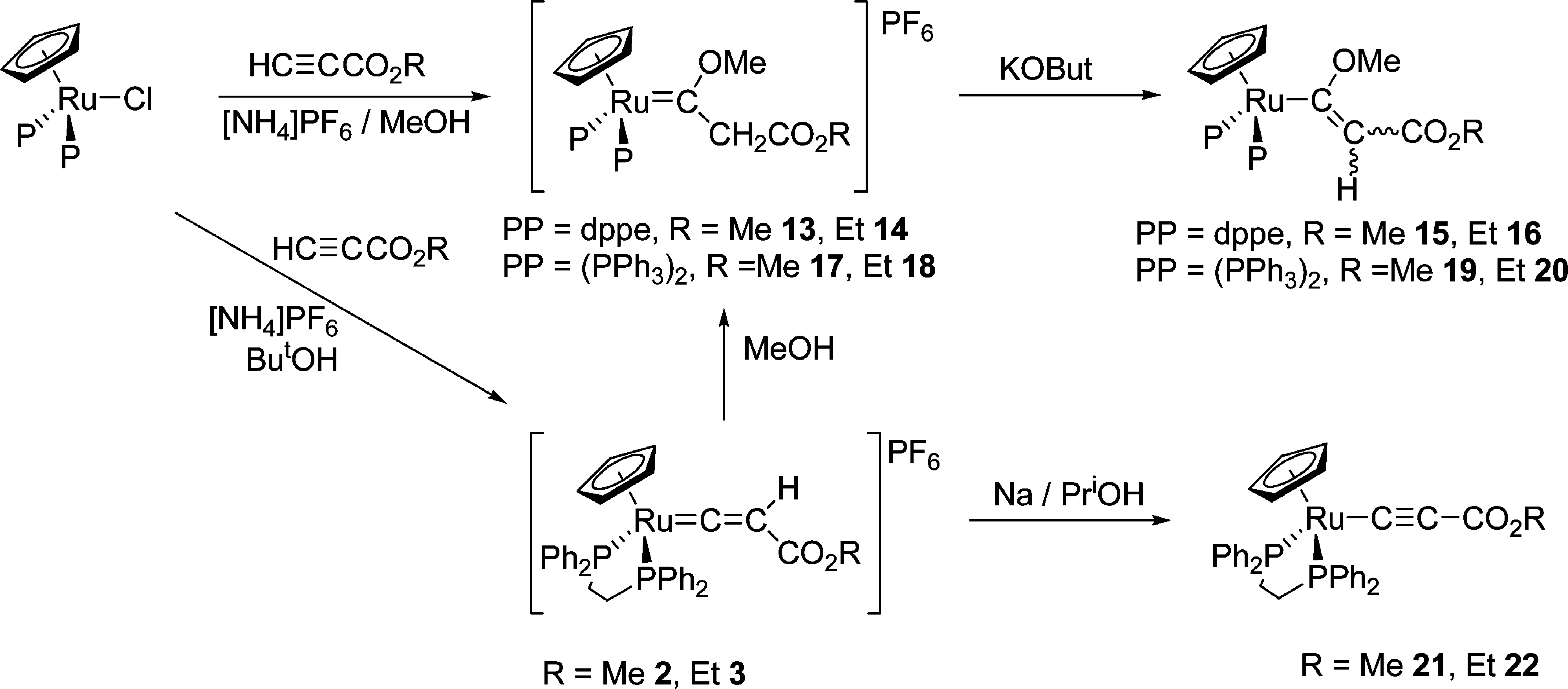
Reactions of Ruthenium Complexes with Propiolic Acid and Esters
CAS number: 474-07-7
Brazilin is a organic heterotetracyclic compound that is a red pigment obtained from the wood of Caesalpinia echinata (Brazil-wood) or Caesalpinia sappan (sappan-wood). It has a role as a plant metabolite, a histological dye, an antineoplastic agent, a biological pigment, an anti-inflammatory agent, an apoptosis inducer, an antioxidant, an antibacterial agent, a NF-kappaB inhibitor and a hepatoprotective agent. It is an organic heterotetracyclic compound, a member of catechols and a tertiary alcohol.
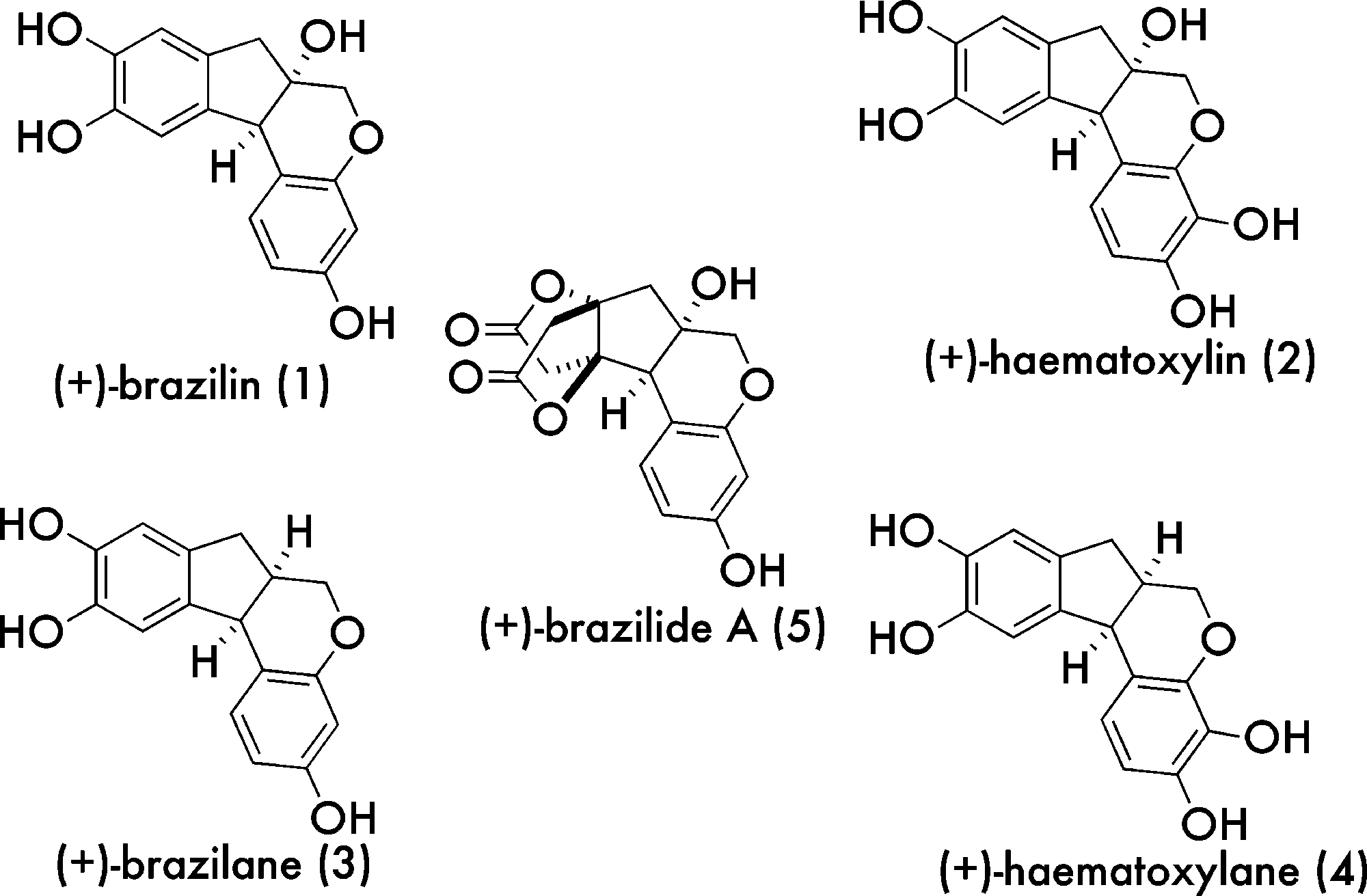
Brazilin family of natural products: (+)-Hematoxylin, (+)-Brazilane, (+)-Brazilide A, (+)-haematoxylane.
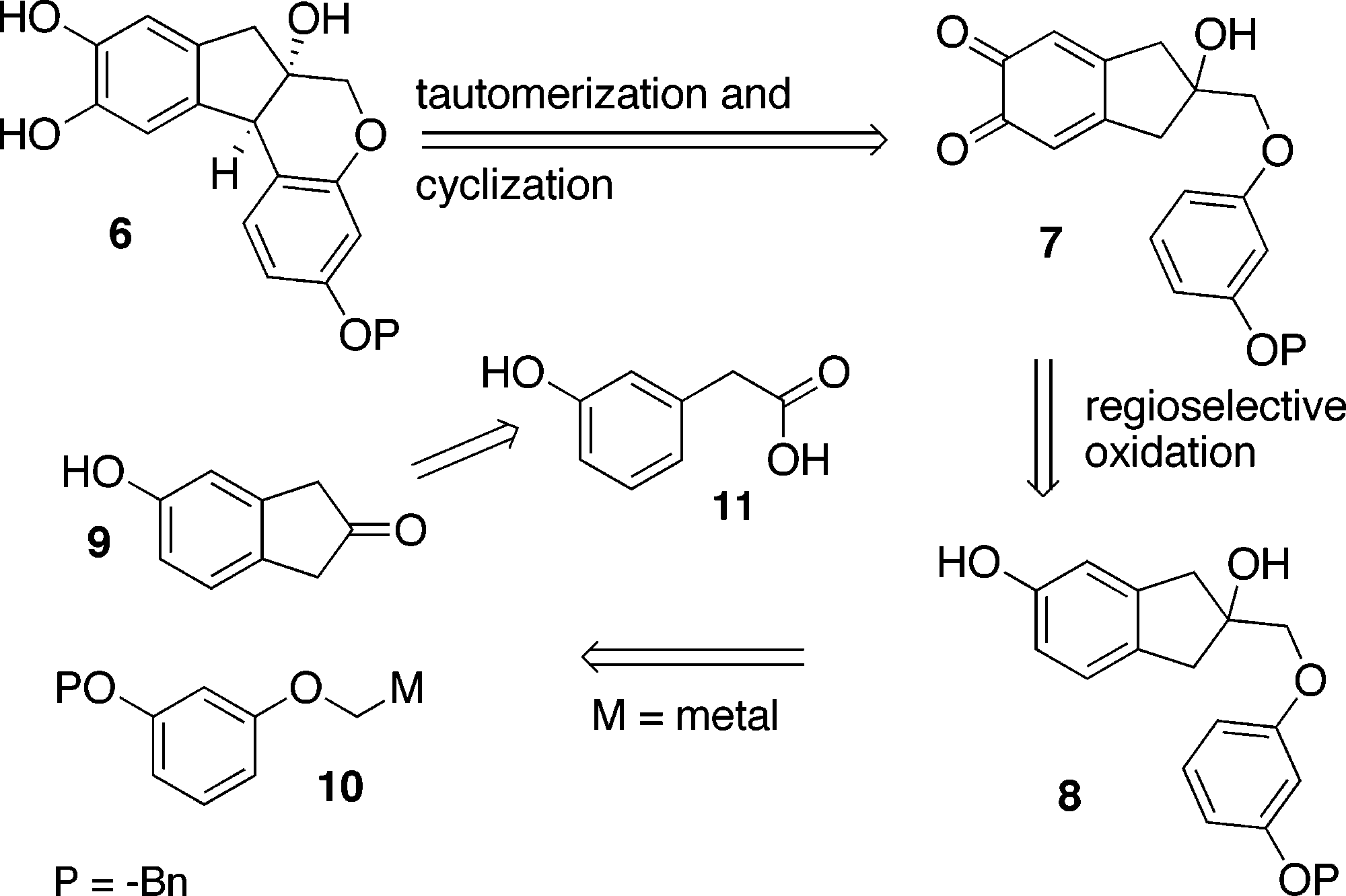
Retrosynthesis of Brazilin (1)
CAS number: 474779-75-4
Perophoramidine is a complex, halogenated natural product isolated from a Philippine marine organism (ascidian) in 2002. It features a unique six-ring structure with two contiguous, or vicinal, quaternary centers and contains bromine, chlorine, and amidine groups. Perophoramidine exhibits cytotoxic activity against colon cancer cells by inducing apoptosis and has been the subject of significant interest in the field of synthetic organic chemistry for its complex structure.
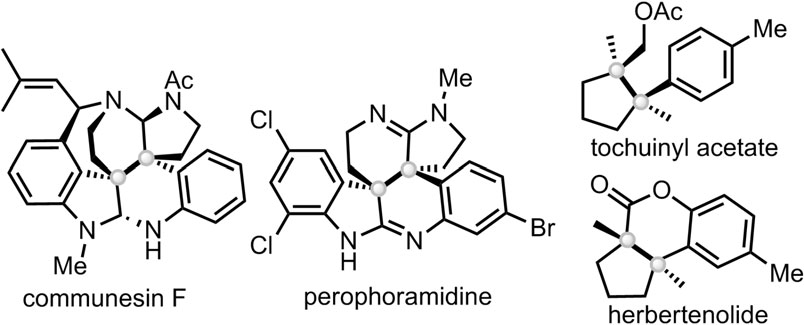
Natural products bearing both stereoisomers of contiguous quaternary all-carbon centers: Communesin F, Perophoramidine, Tochuinyl acetate, Herbertenolide.