Chemicals list & Research Gallery
CAS number: 29218-27-7
Toloxatone is an antidepressant agent, the first ever use of which was in France, 1984. It acts as a selective and reversible inhibitor of monoamine oxidase-A (MOA).
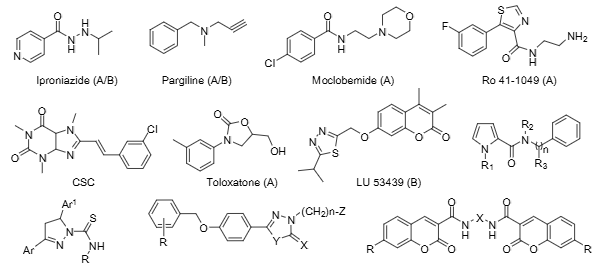
Some representative structures of MAO inhibitors used in research or clinical practice: Iproniazide, Pargiline, Moclobemide, Ro 41-1049, CSC, Toloxatone, LU-53439.
CAS number: 2923-28-6
Silver triflate is a highly effective catalyst in organic reactions, promoting reactions like Friedel-Crafts reactions, nucleophile-alkene cyclizations, and esterifications. Silver triflate can be prepared from the reaction of trifluoromethanesulfonic acid with silver oxide or silver carbonate.
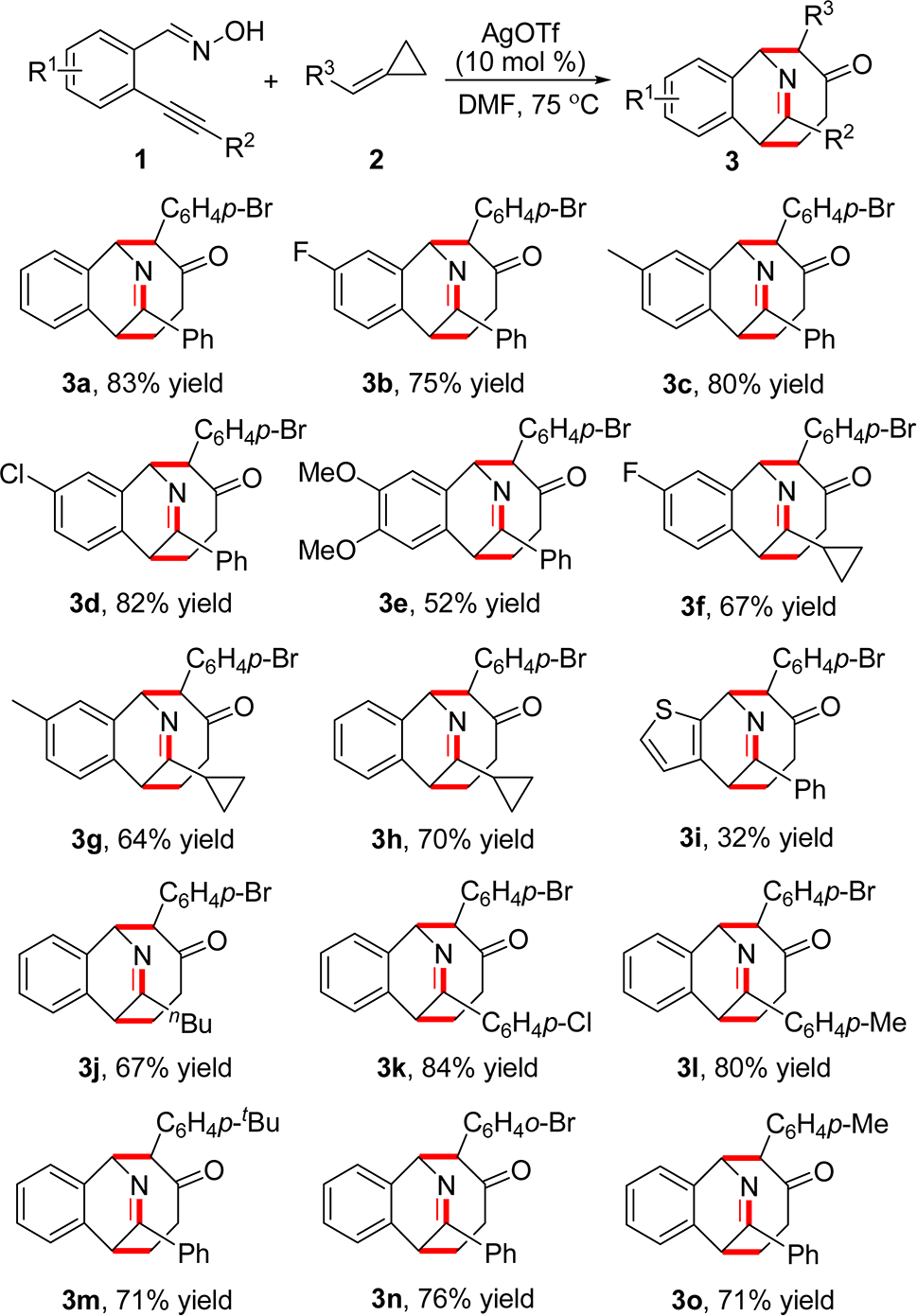
Scope Investigation for the Silver Triflate-Catalyzed Tandem Reaction of 2-Alkynylbenzaldoxime 1 with Alkylide-necyclopropane 2
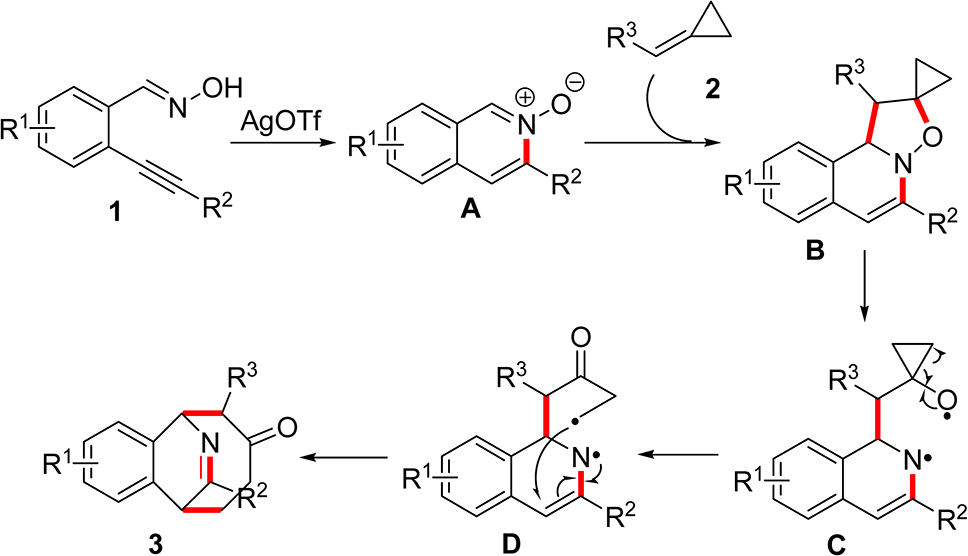
Possible Mechanism for the Silver Triflate-Catalyzed Tandem Reaction of 2-Alkynylbenzaldoxime 1 with Alkylide-necyclopropane 2
CAS number: 2943-56-8
Thymine glycol is a hydroxypyrimidine. It is functionally related to a thymine.
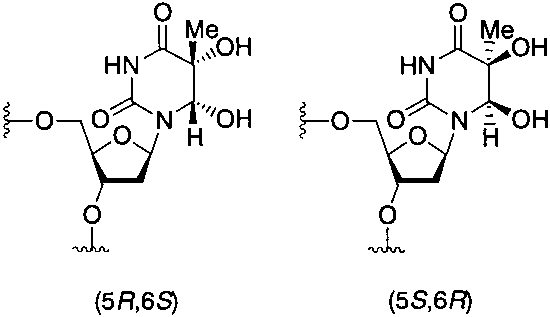
Structures of the two isomers of thymine glycol.
CAS number: 2961-80-0
2-Cyclopropen-1-one is a highly strained, three-membered cyclic ketone. As the smallest member of the cyclopropenone family, it consists of a cyclopropene ring bearing a carbonyl group, making it both aromatic and electrophilic.

Photochemical decarbonylation of a cyclopropenone caged BCN probe (photo-DMBO) activates reactivity toward tetrazine-containing proteins, conferring spatial and temporal control over protein labeling.
CAS number: 29761-81-7
3-Pyridinyl is a substituted pyridine ring in which a functional group is attached at the third carbon of the six-membered aromatic nitrogen-containing ring. The parent structure, pyridine, is similar to benzene but contains one nitrogen atom replacing a CH group, imparting basicity and unique reactivity. In the 3-pyridinyl configuration (also called meta-pyridyl), the substituent is bonded to the carbon atom adjacent to the nitrogen (but not directly next to it), influencing the molecule's electronic and steric properties. This moiety is commonly found in pharmaceuticals, agrochemicals, and ligands due to its ability to participate in hydrogen bonding, coordination chemistry, and π–π interactions.
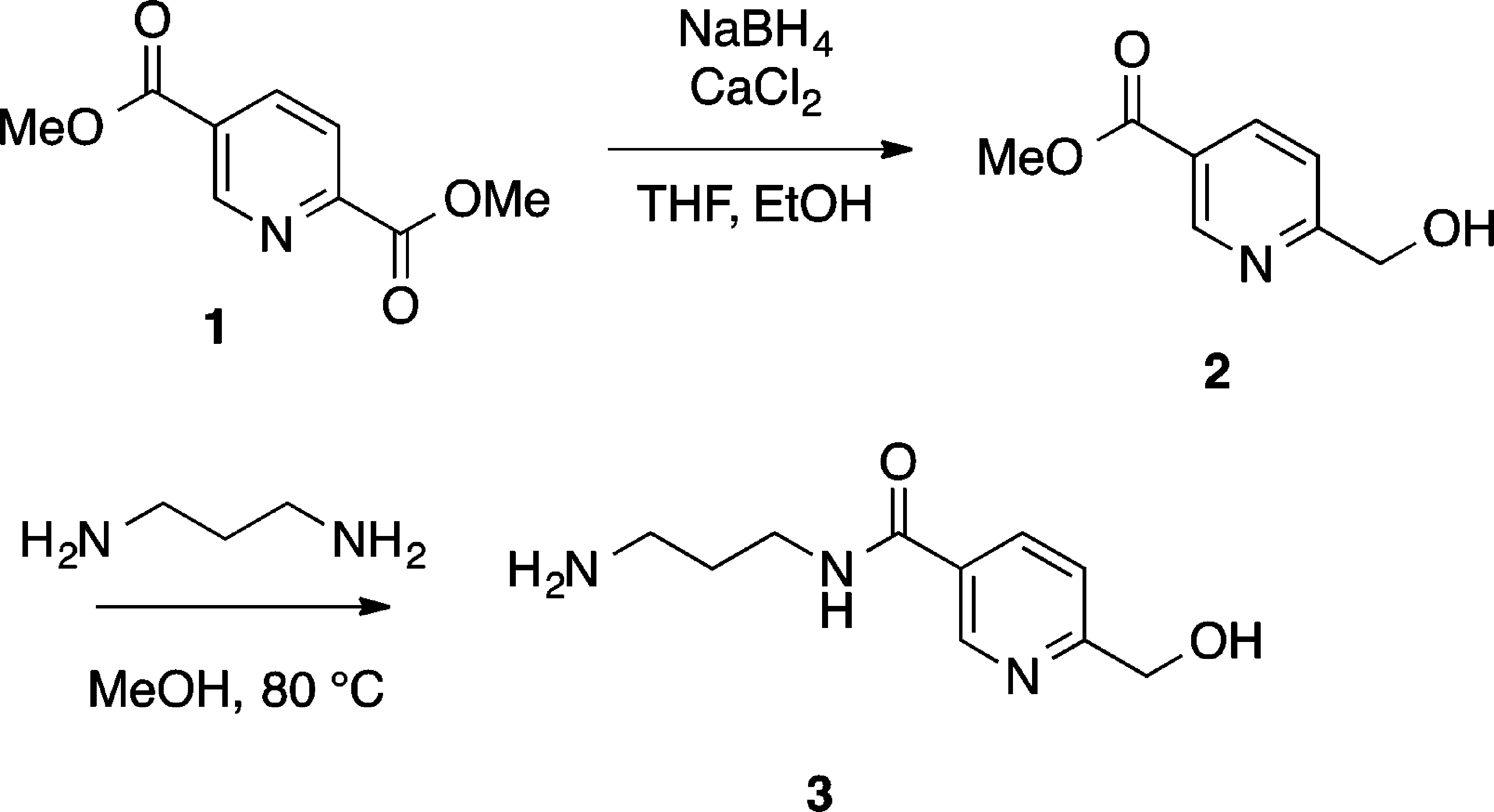
Synthesis of Pyridine Precursor 3
CAS number: 298-12-4
Glyoxylic acid is a 2-oxo monocarboxylic acid that is acetic acid bearing an oxo group at the alpha carbon atom. It has a role as a human metabolite, an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite and a mouse metabolite. It is a 2-oxo monocarboxylic acid and an aldehydic acid. It is a conjugate acid of a glyoxylate.
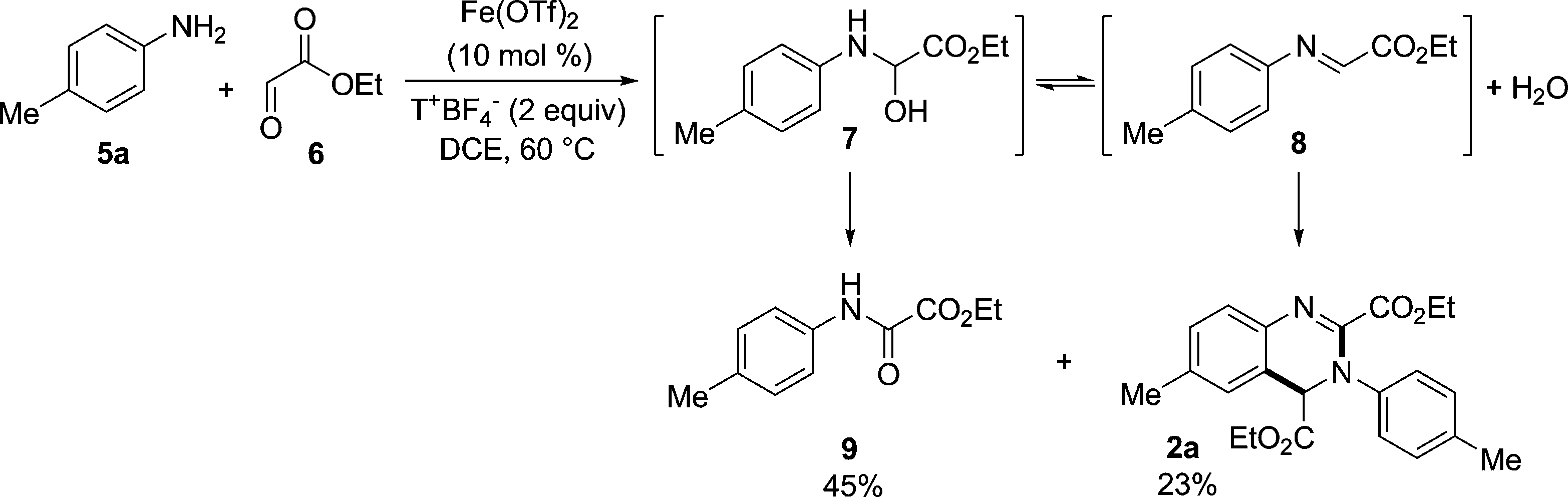
One-Pot Reaction between Aniline 5a and Glyoxalate 6
CAS number: 29976-75-8
6-Methylflavone is a member of flavones.
![Competition curves of naltrexone (◼) and compounds 8 (△), 9 (▼), 10 (+), 12 (▲), 15 (◇), 31 (○) and 32 (●) for [3H]-DAMGO binding to Wistar rat forebrain membranes.](http://www.wlxkc.cn/picture/2127528_10.png)
Competition curves of naltrexone (◼) and compounds 8 (△), 9 (▼), 10 (+), 12 (▲), 15 (◇), 31 (○) and 32 (●) for [3H]-DAMGO binding to Wistar rat forebrain membranes.
CAS number: 300657-03-8
BMS-309403 is a synthetic, cell-permeable, and orally active chemical compound that acts as a potent and selective inhibitor of fatty acid binding protein 4 (FABP4), also known as aP2. It is being researched for its potential in treating conditions like type 2 diabetes and atherosclerosis due to its ability to reduce inflammation and improve insulin sensitivity.
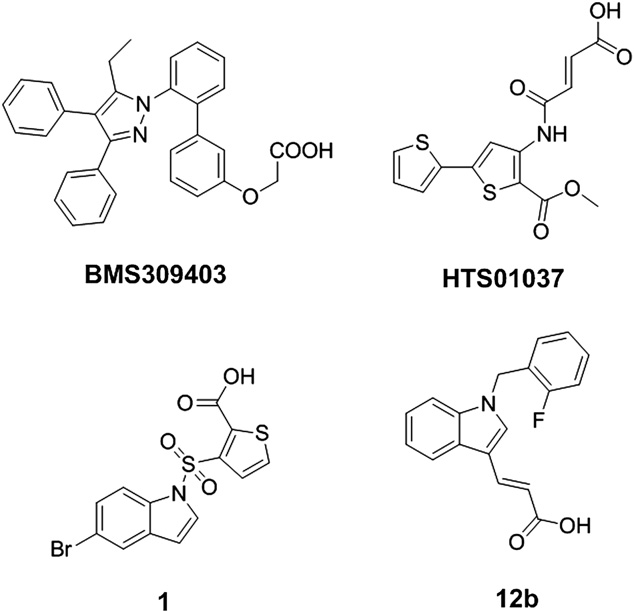
Structure of BMS309403, HTS01037, 1 and 12b.
CAS number: 301-02-0
Oleamide is a fatty amide derived from oleic acid. It has a role as a human metabolite and a plant metabolite.
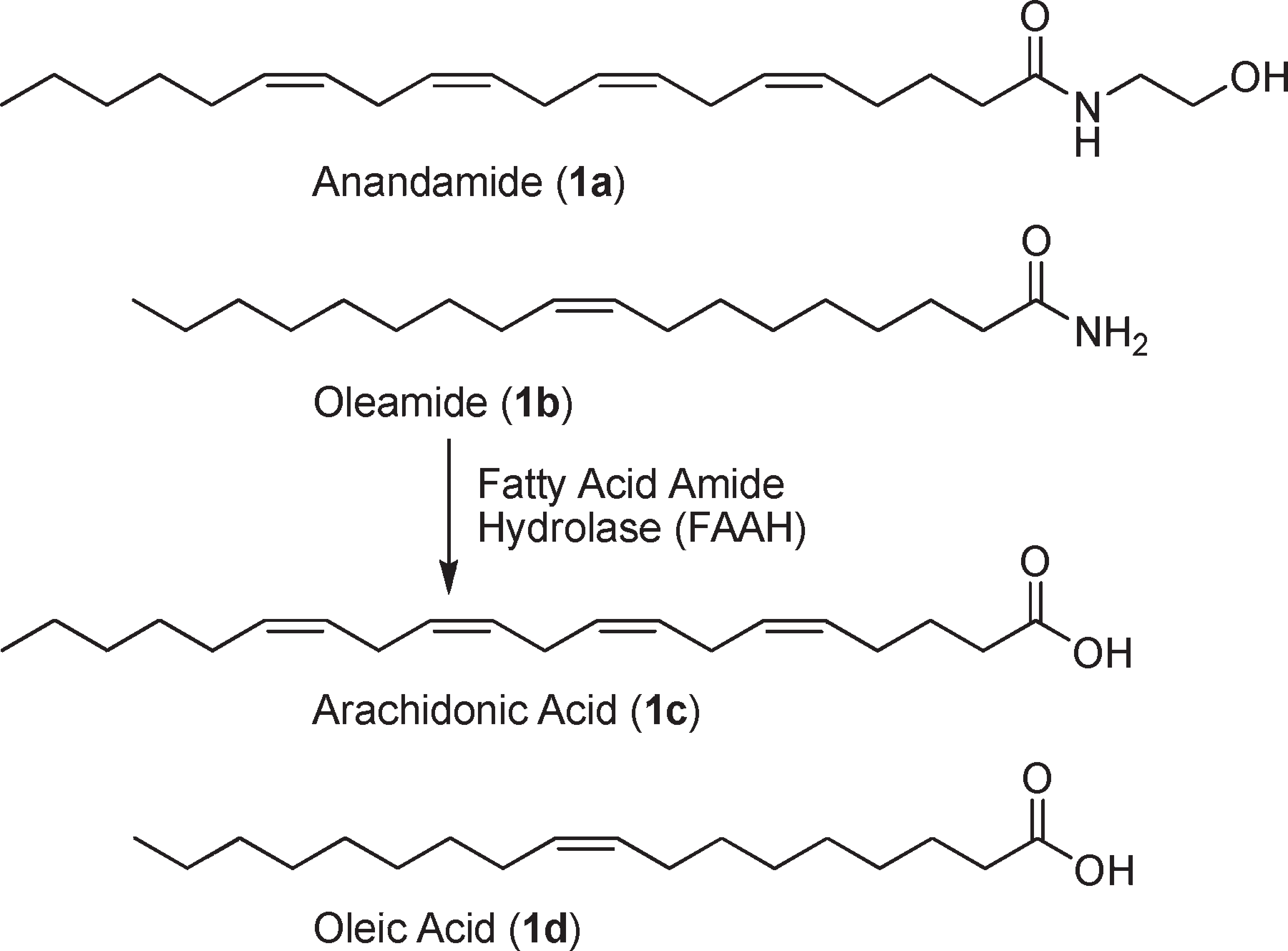
Substrates of fatty acid amide hydrolase (FAAH).
CAS number: 302-01-2
Hydrazine, anhydrous appears as a colorless, fuming oily liquid with an ammonia-like odor. It can cause cancer according to an independent committee of scientific and health experts.
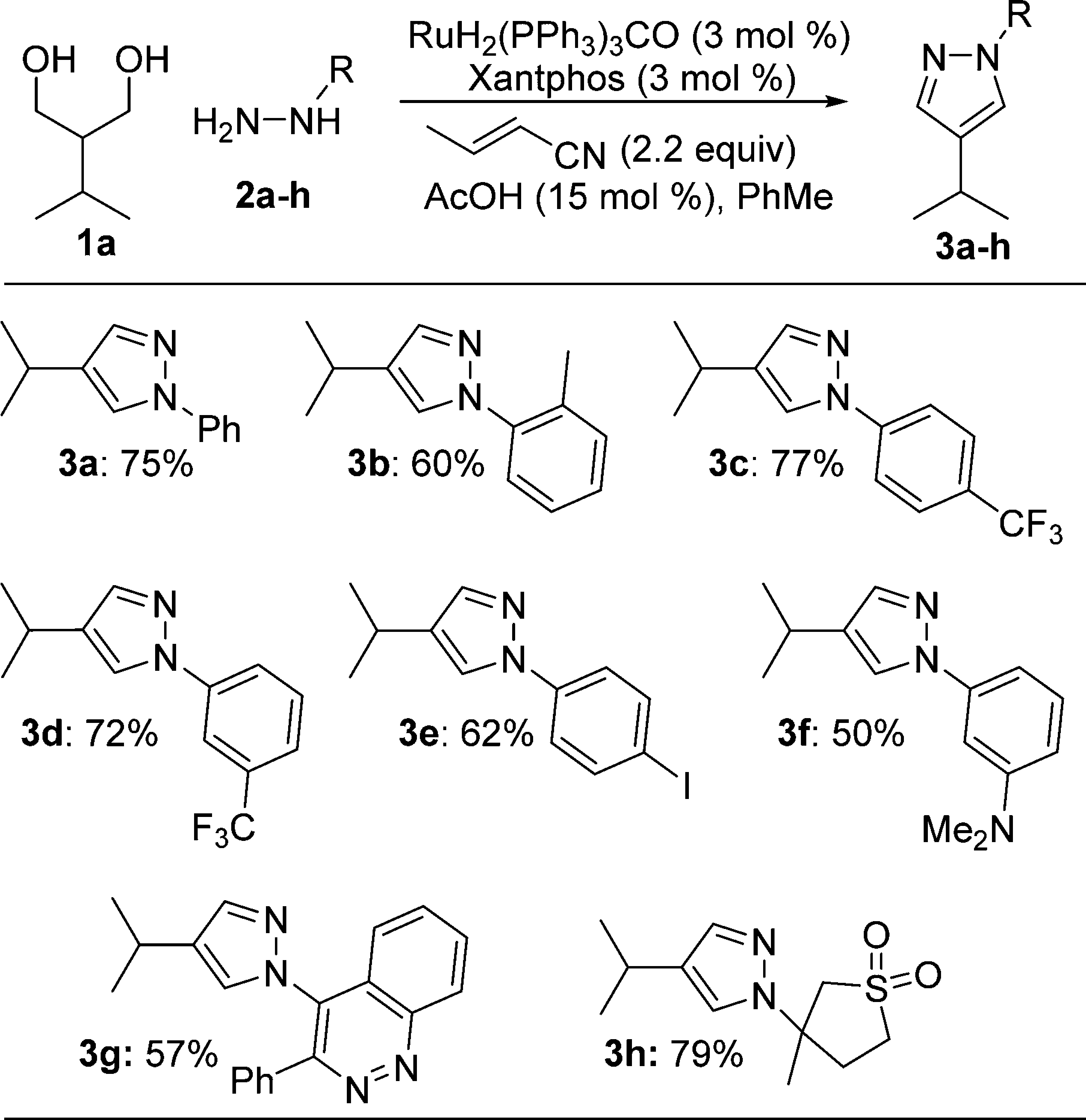
Hydrazine Scope
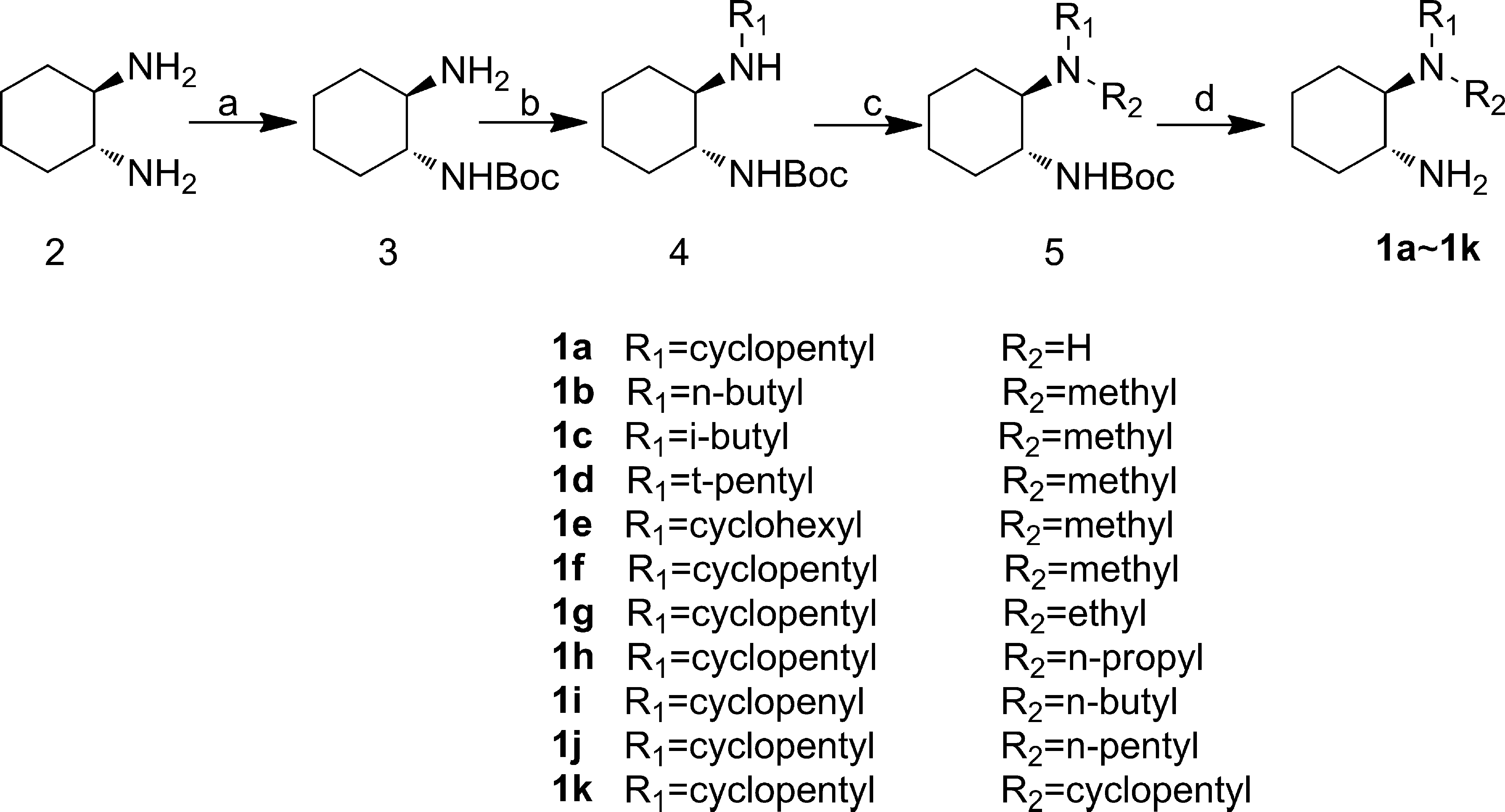
Modified diamine catalysts were synthesized starting from the commercial chiral diamine 2. All catalysts except 1a and 1k were easily prepared from 2 in a 4-step sequence as shown in Scheme 2.
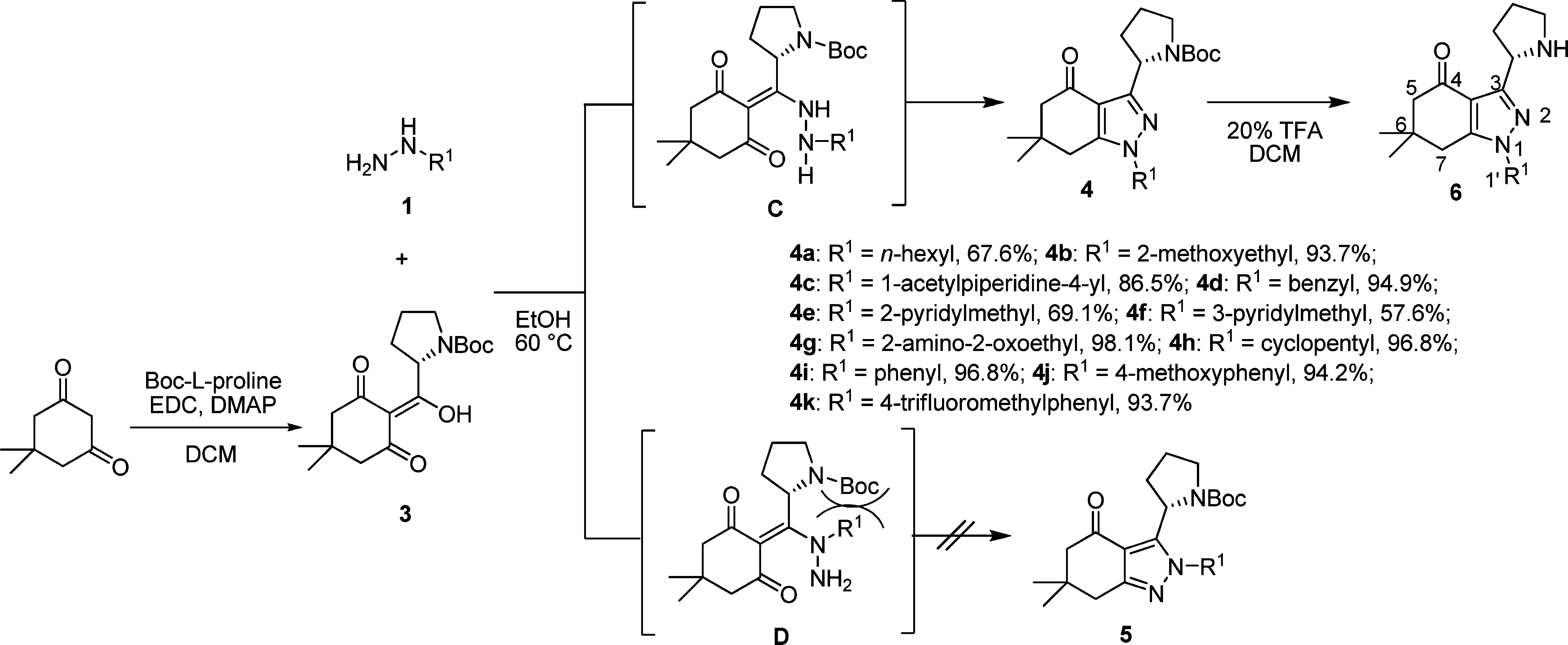
Regioselective Synthetic Pathway for 1,3-Disubstituted Tetrahydroindazolones Using Hydrazines 1 and 2-(Hydroxy(N-Boc-pyrrolidin-2-yl)methylene)-5,5-dimethylcyclohexane-1,3-dione 3