Chemicals list & Research Gallery
CAS number: 2761424-76-2
mGluR5 antagonist-1 is an orally active mGluR5 antagonist with an IC50 value of 11.5 nM. mGluR5 antagonist-1 has anti-depressant effect. mGluR5 antagonist-1 can be used for the study of depressive disorder.
![Autoradiography on horizontal rat brain slices, indicating heterogeneous distribution of activity with highest uptake in mGluR5-rich regions. The first and second rows represent application of 1 and 10 nM [18F]-16, respectively, and the first and second column represent baseline and blocking conditions, respectively.](http://www.wlxkc.cn/picture/5367115_16.png)
Autoradiography on horizontal rat brain slices, indicating heterogeneous distribution of activity with highest uptake in mGluR5-rich regions. The first and second rows represent application of 1 and 10 nM [18F]-16, respectively, and the first and second column represent baseline and blocking conditions, respectively.
CAS number: 2781-85-3
Cyclopropene is a cycloalkene that consists of cyclopropane having a double bond in the ring. The parent of the class of cyclopropenes.
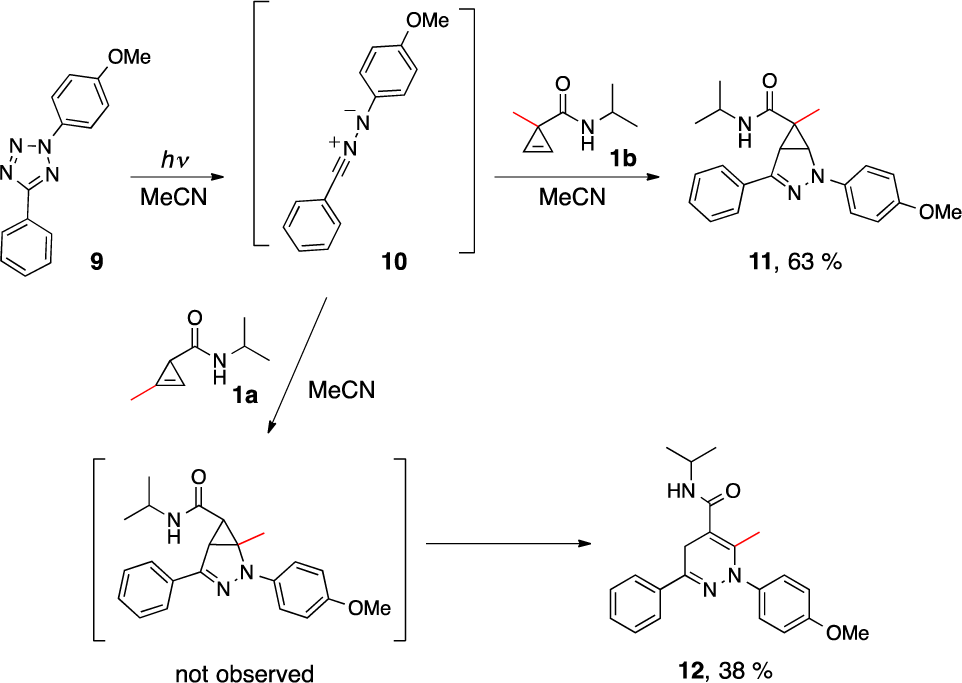
Cyclopropene reacts with nitrile imine to form a stable cyclic adduct
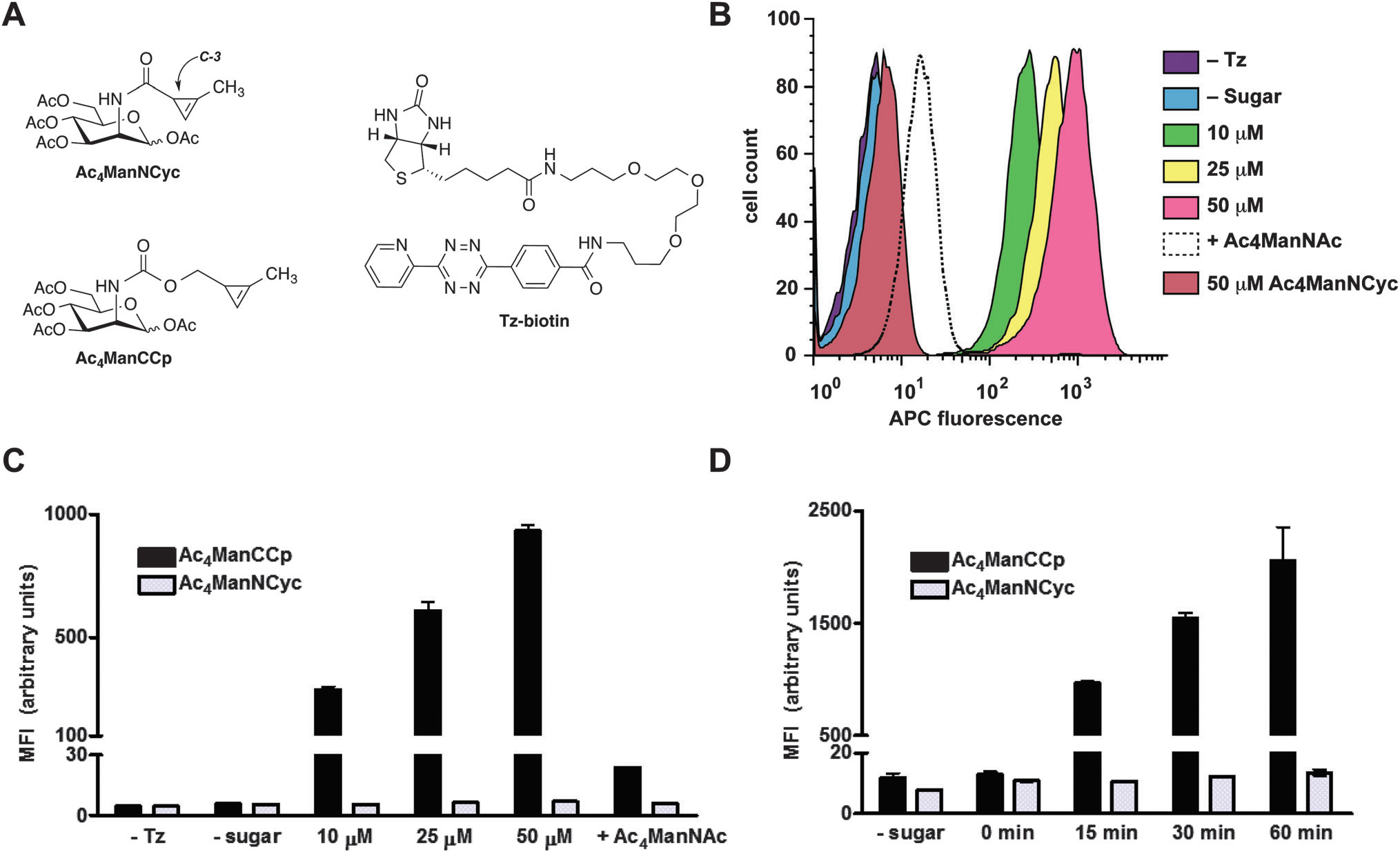
Cyclopropene-modified ManNAc derivatives can be metabolically incorporated onto cell surfaces and covalently detected with tetrazine probes.
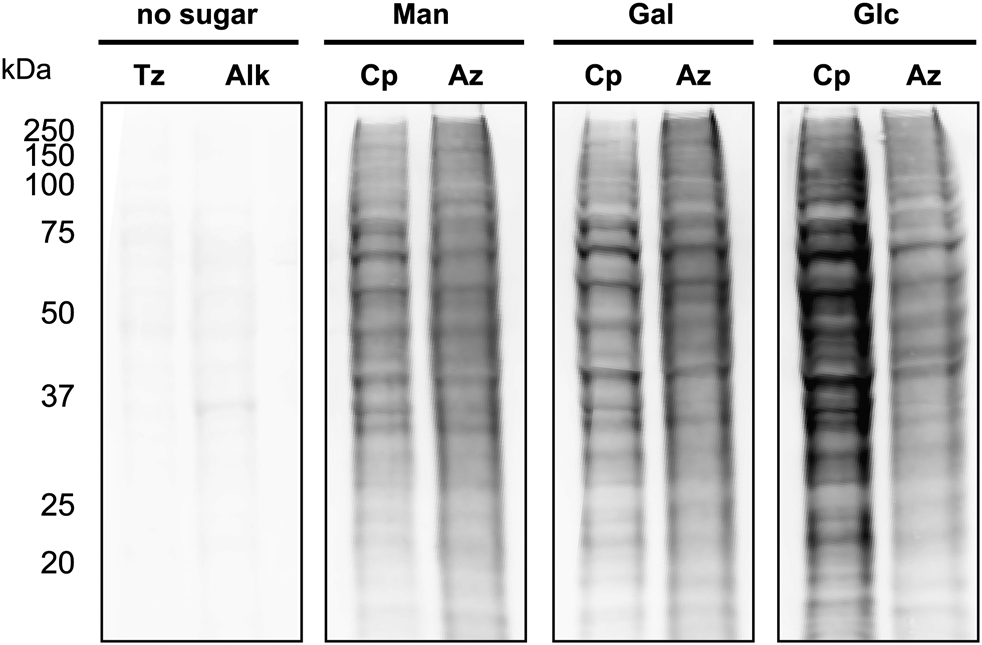
Carbamate-linked cyclopropene sugars label cellular glycoproteins.
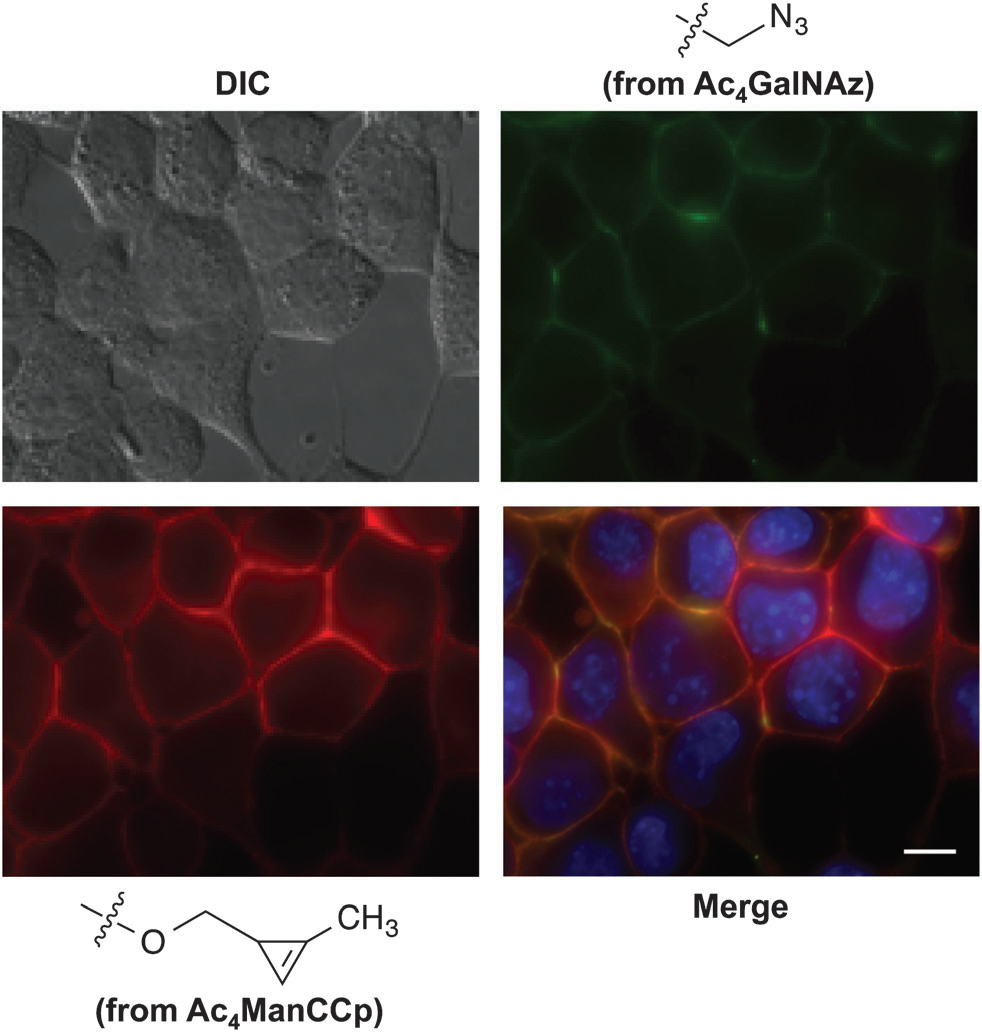
Distinct metabolic targets can be simultaneously imaged using cyclopropene and azido reporters.
CAS number: 27988-97-2
Tetrazole is a heterocyclic compound containing a carbon atom and four nitrogen atoms in a five-membered ring.
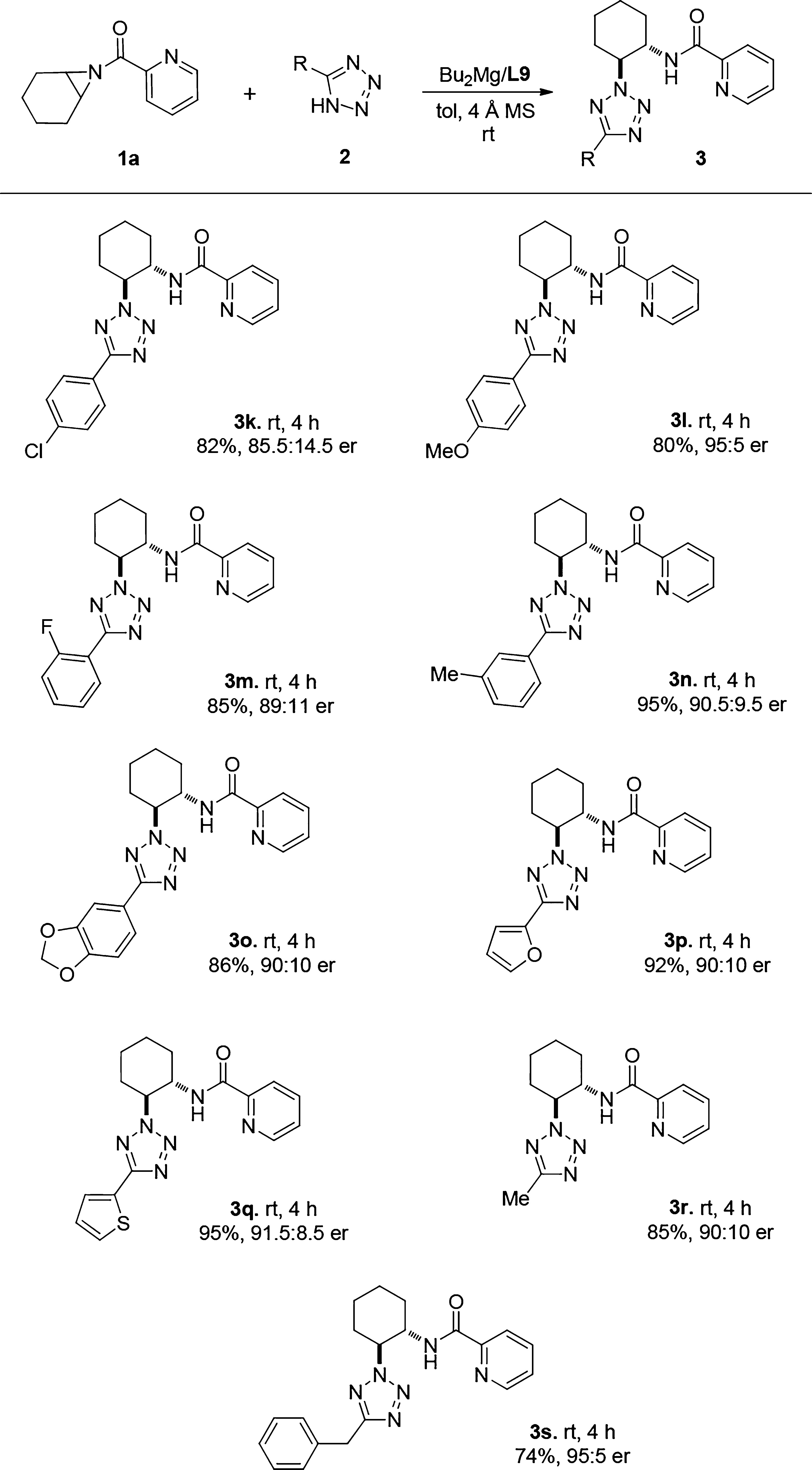
Substrate Scope of the Tetrazole-Involved Desymmetrization Reaction
CAS number: 281-54-9
Calix[4]arene is a calixarene.
![Scaffolds of the homologue calix[4]arene (1), calix[4]naphthalene (2) and calix[4]phenanthrene (3).](http://www.wlxkc.cn/picture/3834702_01.png)
Scaffolds of the homologue calix[4]arene (1), calix[4]naphthalene (2) and calix[4]phenanthrene (3).
CAS number: 282089-49-0
N-(1,3-benzodioxol-5-ylmethyl)-7-methoxy-2-oxo-8-pentoxy-1H-quinoline-3-carboxamide is a member of quinolines and an aromatic amide.
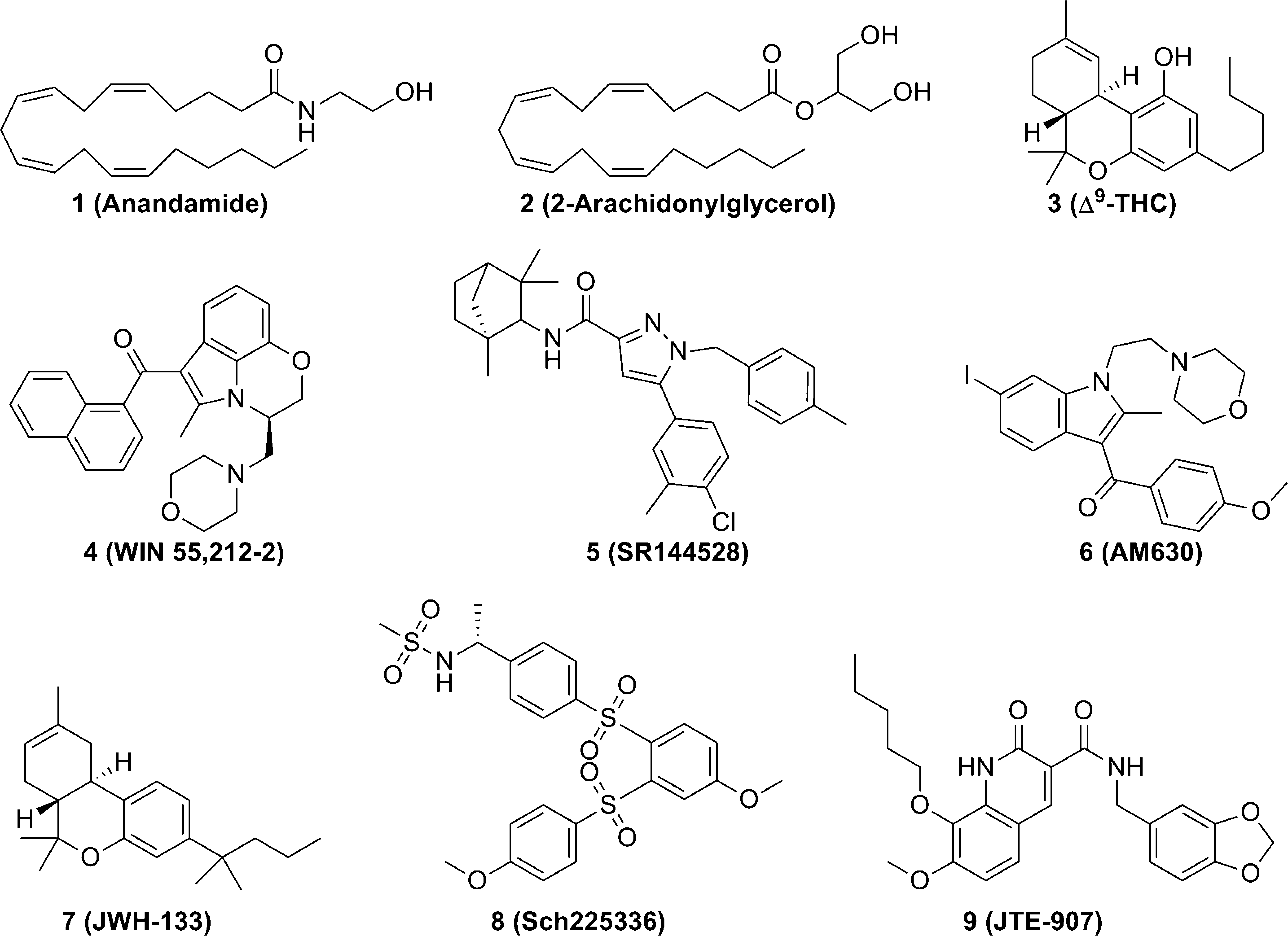
Representative cannabinoids with various chemical scaffolds: Anandamide, 2-Arachidonylglycerol, Win-55212-2, SR 144528, JWH-133, JTE-907.
CAS number: 2835-77-0
2-Aminobenzophenone is an organic chemical compound, specifically a derivative of benzophenone. It's also known as 2-Benzoylaniline or o-Aminobenzophenone.
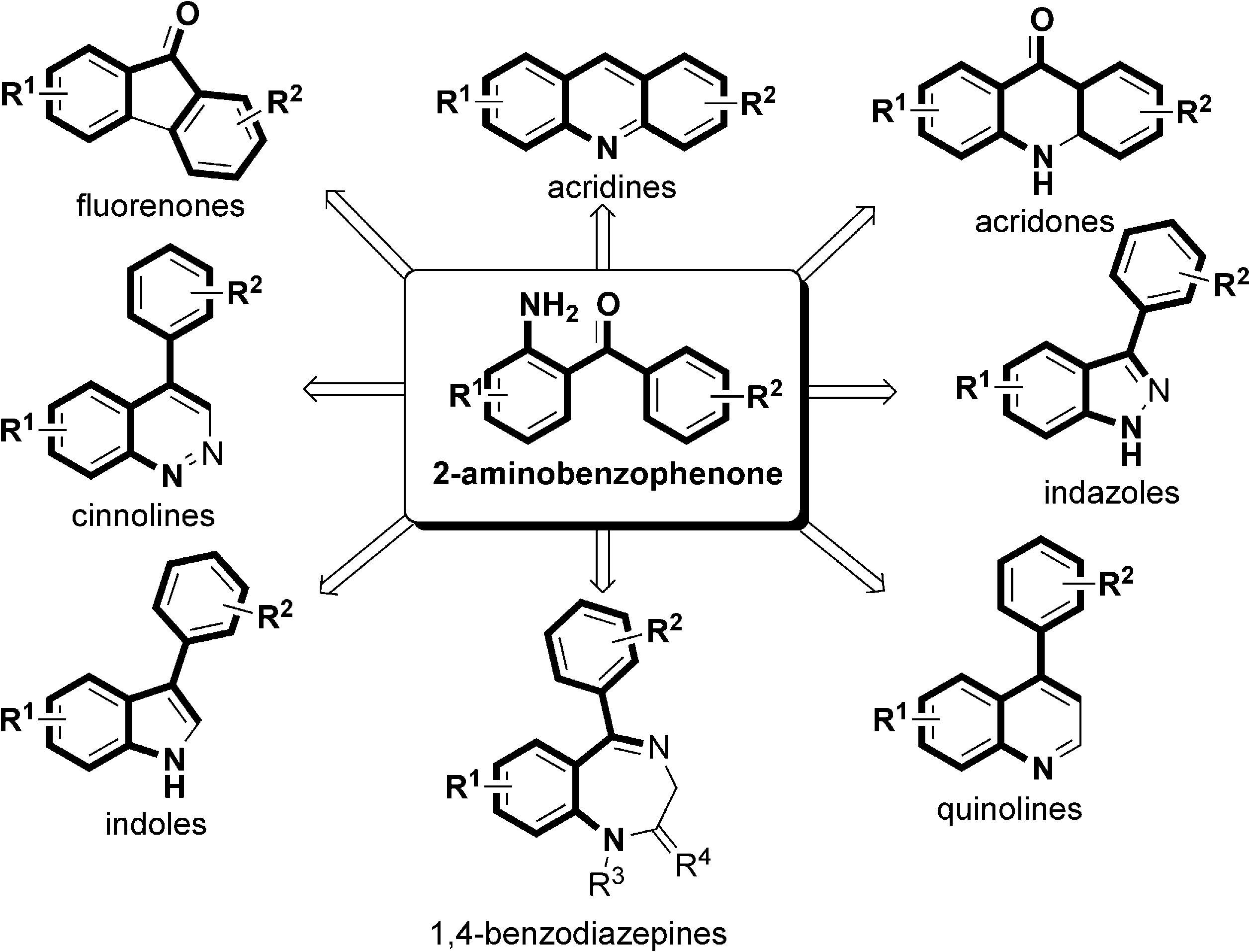
Some medicinally useful heterocycles derived from 2-aminobenzophenones.
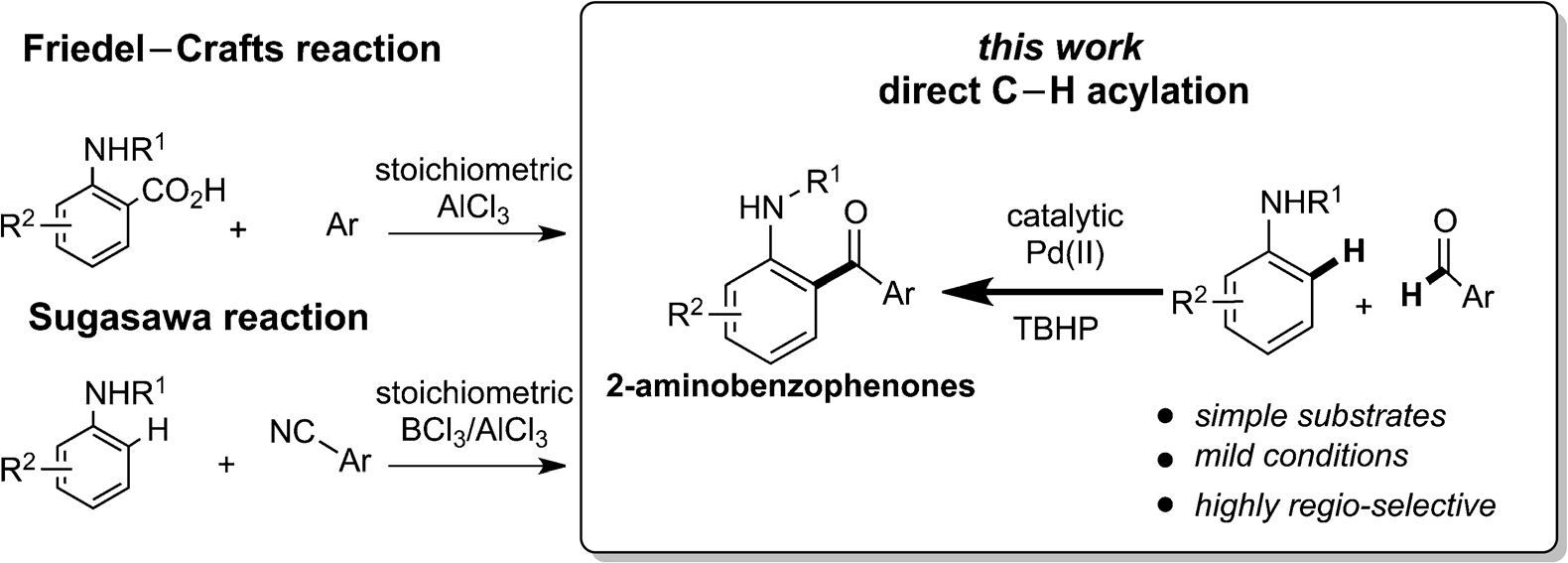
Synthesis of 2-aminobenzophenones.
CAS number: 28350-87-0
2-pyrroline is a pyrroline.
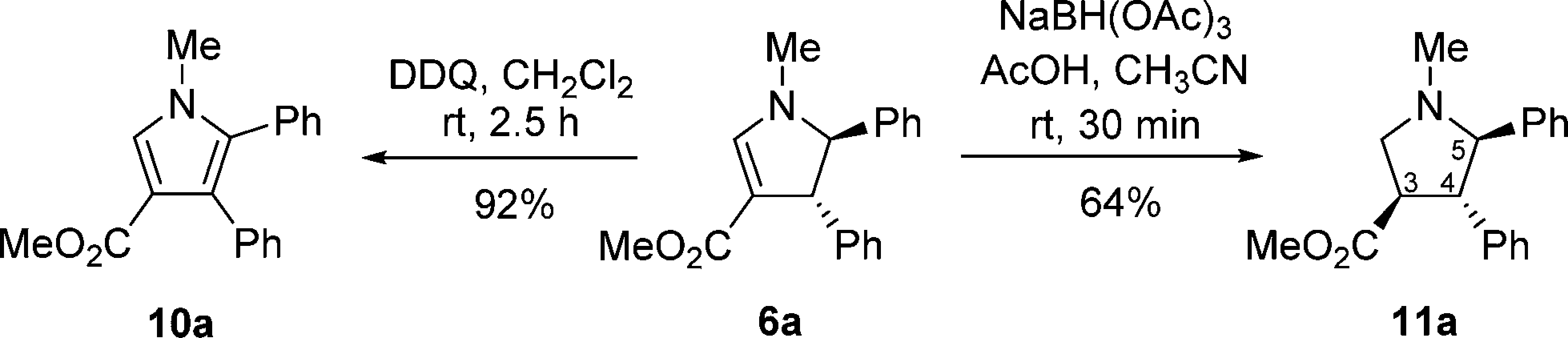
Synthetic Derivatization of Dihydropyrroles
CAS number: 28395-03-1
Bumetanide is a member of the class of benzoic acids that is 4-phenoxybenzoic acid in which the hydrogens ortho to the phenoxy group are substituted by butylamino and sulfamoyl groups. Bumetanide is a diuretic, and is used for treatment of oedema associated with congestive heart failure, hepatic and renal disease. It has a role as a diuretic and an EC 3.6.3.49 (channel-conductance-controlling ATPase) inhibitor. It is a sulfonamide, an amino acid and a member of benzoic acids.
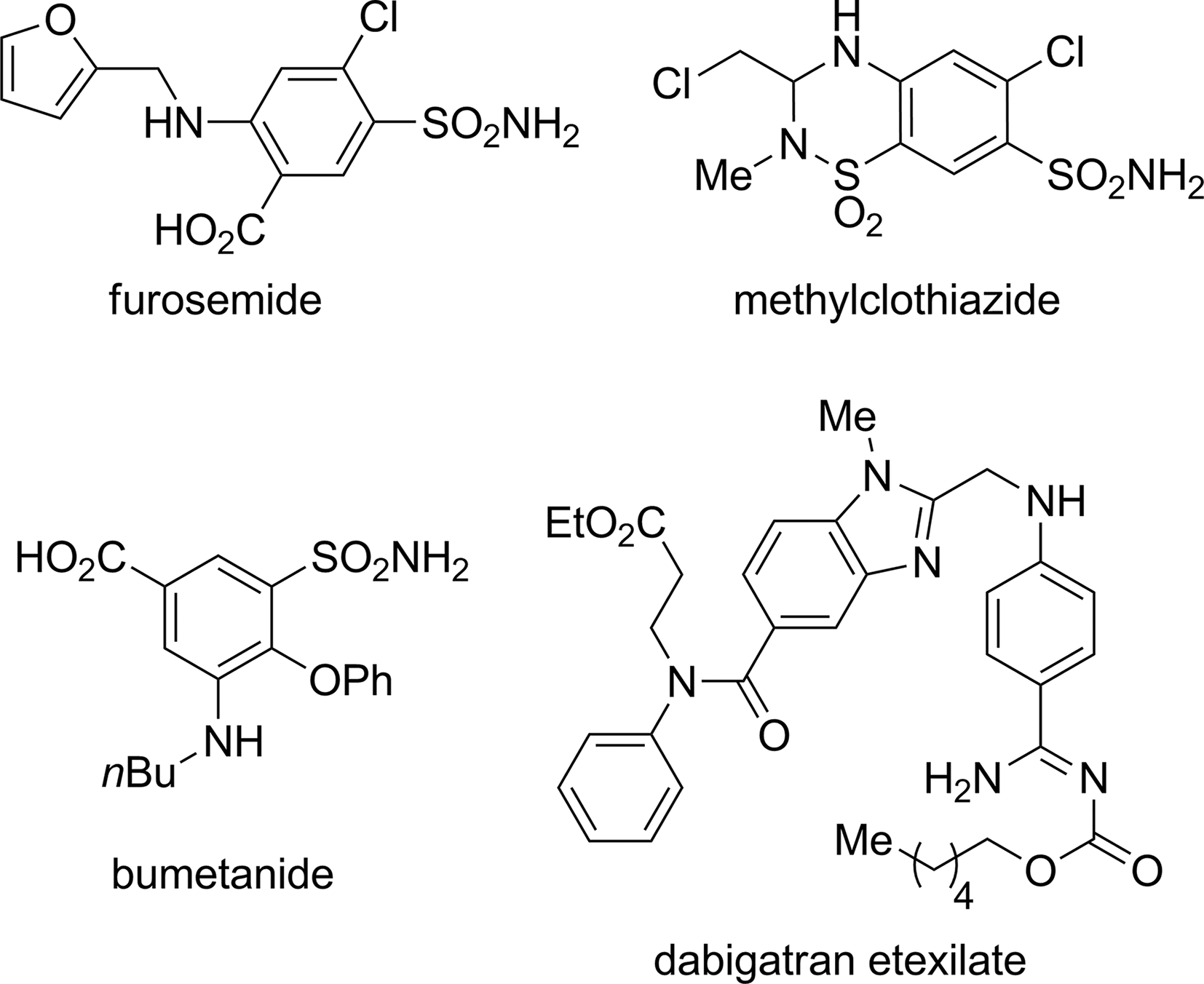
Some aniline-containing pharmaceuticals: furosemide, methylclothiazide, bumetanide
CAS number: 28638-58-6
Cyclopentene, 1-ethenyl-, also known as 1-vinylcyclopentene, is a cyclic alkene containing a cyclopentene ring with a vinyl group (ethenyl group) attached to it.
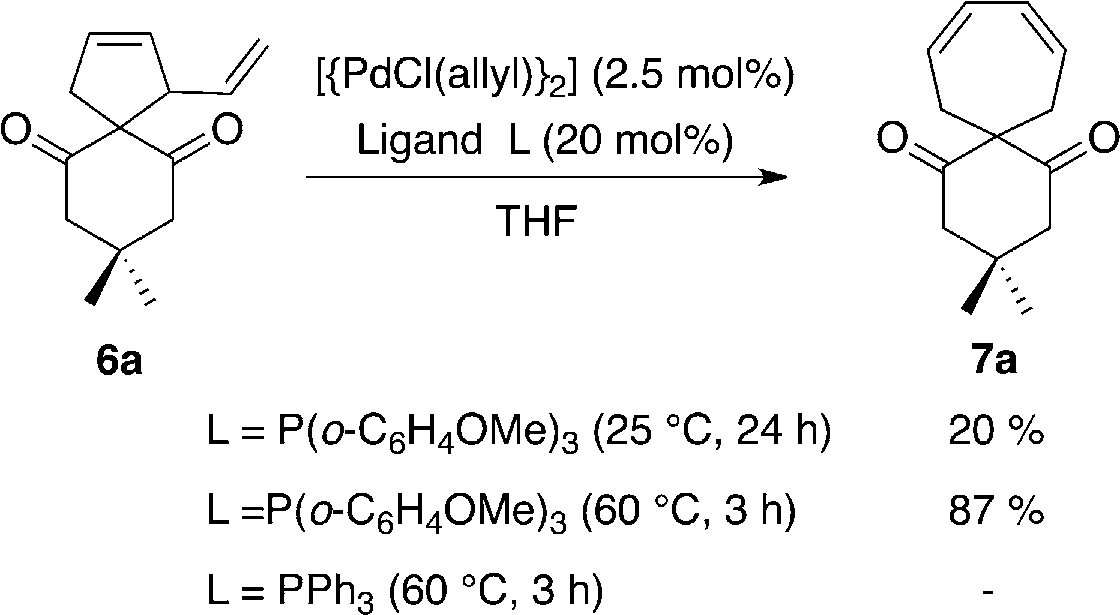
Transformation of vinylcyclopentene 6a into cycloheptadiene 7a.
CAS number: 28647-38-3
Diazynium is a nitrogen hydride. It is a conjugate base of a diazynediium. It is a conjugate acid of a dinitrogen.
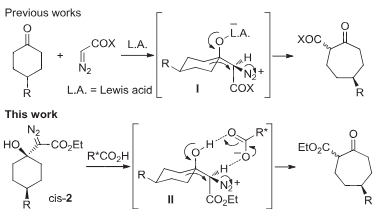
Desymmetrization via diazonium intermediates.