Chemicals list & Research Gallery
CAS number: 24280-93-1
Mycophenolic acid is a potent immunosuppressant agent that inhibits de novo purine biosynthesis. It was derived from Penicillium stoloniferum, and has also shown antibacterial, antifungal and antiviral properties.. Mycophenolic acid is used in immunosuppressive regimens as part of a triple therapy that includes a calcineurin inhibitor (ciclosporin or tacrolimus) and prednisolone.
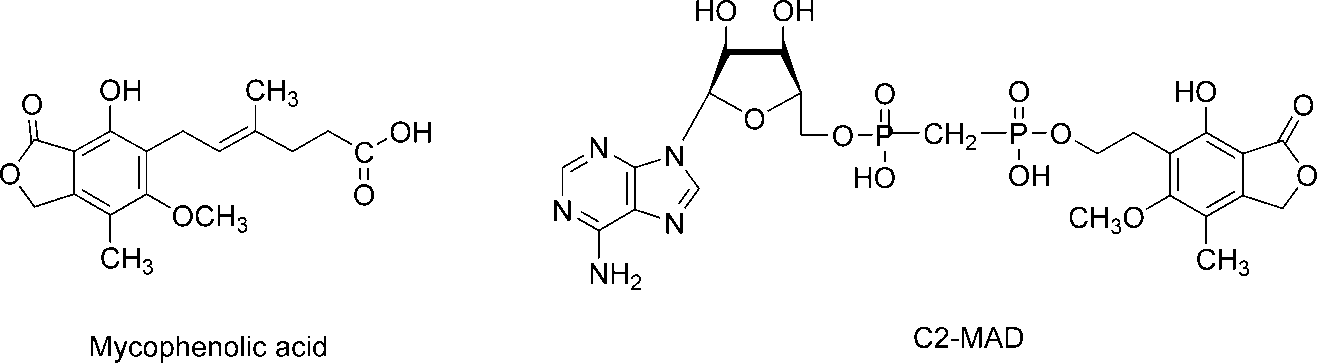
Mycophenolic acid (MPA), one of the most potent inhibitors of human IMPDH (Ki ) 10 nM), binds at the NAD binding domain of the enzyme and is used in clinic as an immunosuppressant. C2-MAD was found to be more potent than MPA in both antileukemic activity and the ability to induce differentiation.
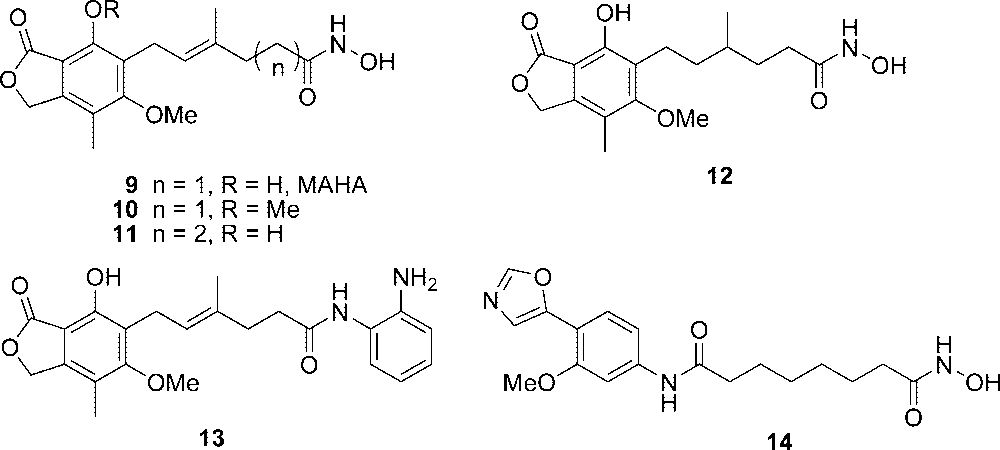
We replaced the carboxylic group of MPA with a hydroxamic acid moiety and synthesized hydroxamic acid analogue 9 (MAHA, Figure 4).
CAS number: 2435-53-2
o-Chloranil, also known as 3,4,5,6-tetrachloro-1,2-benzoquinone, is a yellow crystalline organic compound. Structurally, it is a chlorinated derivative of ortho-benzoquinone, featuring four chlorine atoms substituted at the 3, 4, 5, and 6 positions of the benzene ring. It is a strong oxidizing agent and is used primarily as an intermediate in organic synthesis, particularly in dye manufacturing, pharmaceuticals, and agrochemicals.
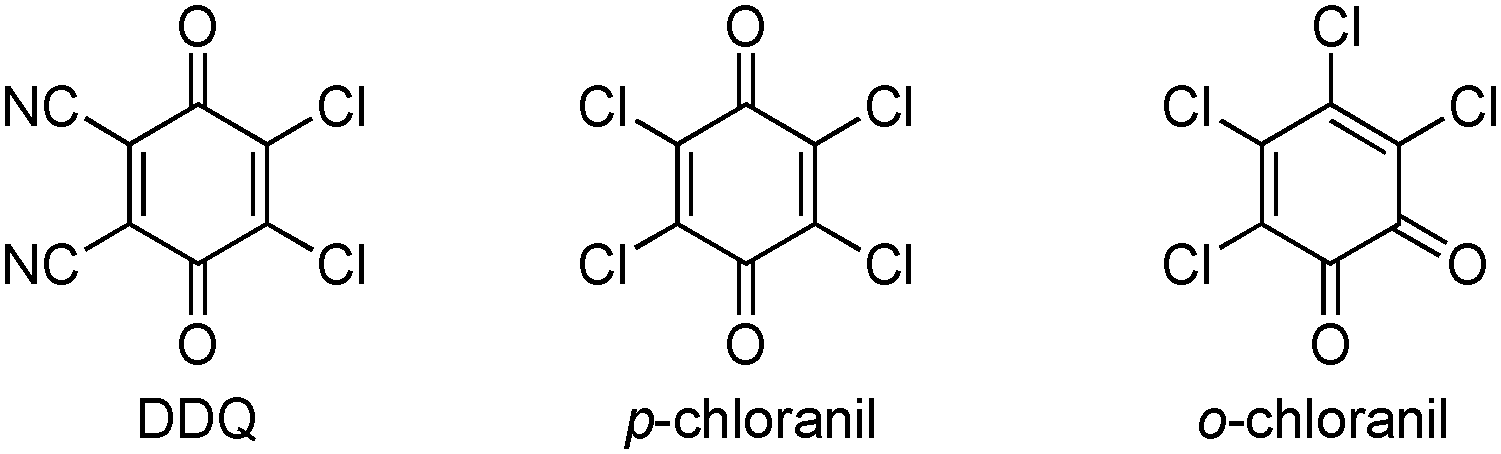
Quinones with high oxidation potential: 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone, p-Chloranil, o-Chloranil
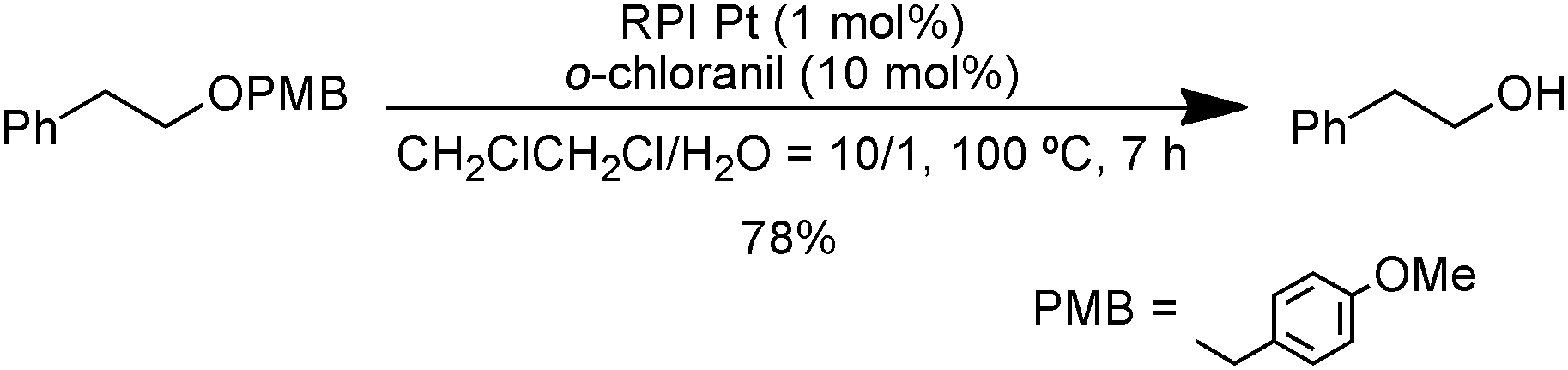
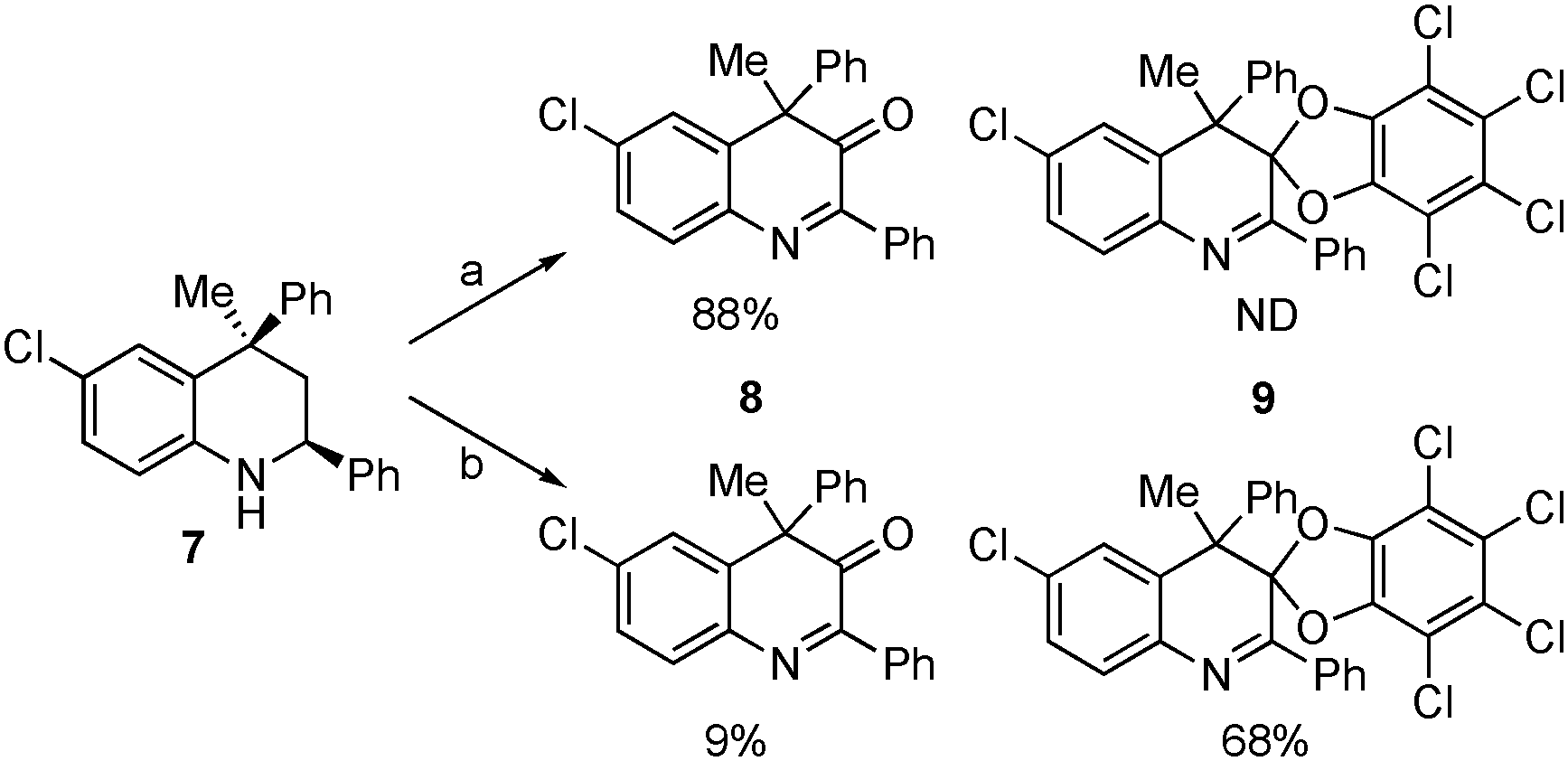
Deprotection of PMB group using catalytic amount of RPI Pt and o-chloranil.
Comparison between a catalytic amount and a stoichiometric amount of o-chloranil in the oxidation of tetrahydroquinoline derivative.
CAS number: 2437-49-2
2,4,6-Tribromoresorcinol is a chemical compound, specifically a brominated phenolic compound. It's primarily used as a flame retardant and as an intermediate in the synthesis of other brominated compounds. It is also known as 1,3,5-Tribromo-2,4-dihydroxybenzene.
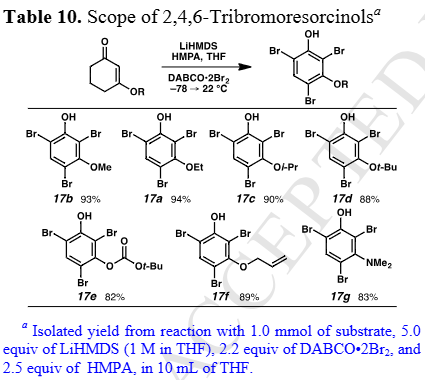
Scope of 2,4,6-Tribromoresorcinols
CAS number: 2438-80-4
Fucose is an unusual sugar that is present in a variety of glycolipids and glycoproteins produced by mammalian cells.
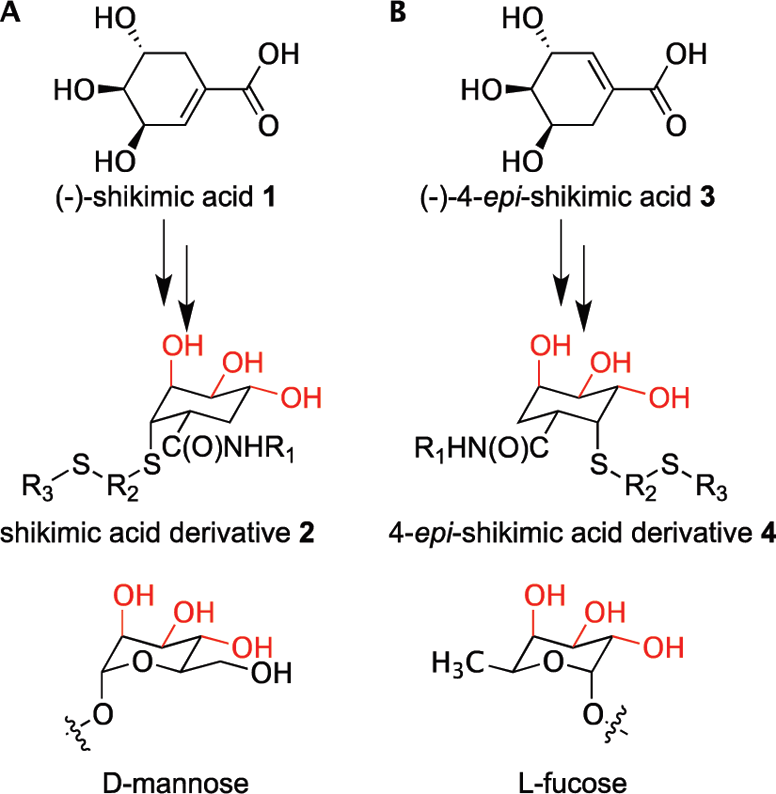
Glycomimetic scaffolds designed to mimic (A) mannose and (B) fucose. Hydroxyl groups typically involved in C-type lectin binding are highlighted in red.
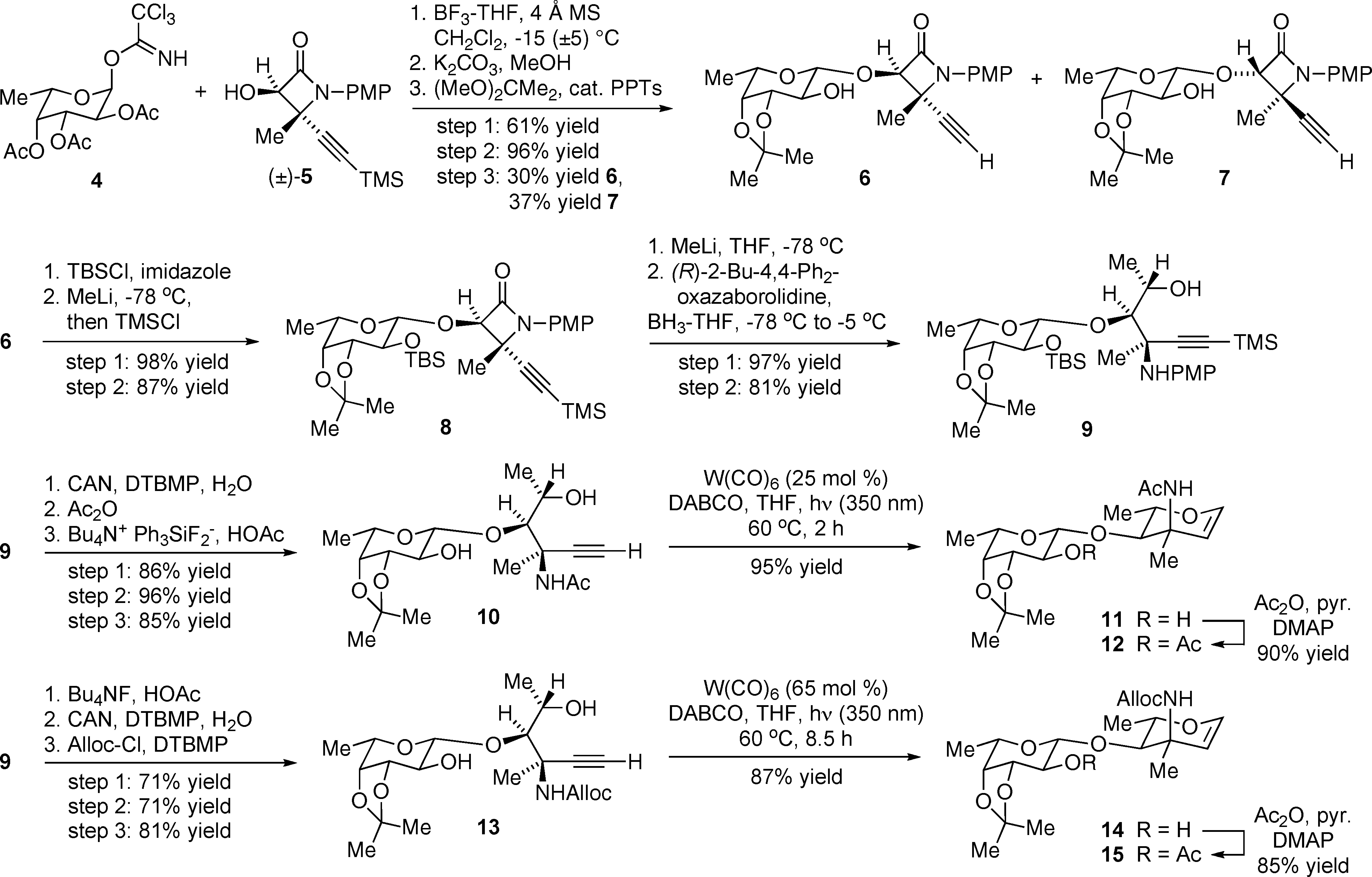
Preparation of Fucose-Saccharosamine Disaccharide Glycals 11-12 and 14-15
CAS number: 244767-67-7
Dapivirine is a diarylpyrimidine non-nucleoside reverse transcriptase inhibitor. Dapivirine has activity against wild-type virus strains and strains harboring different NNRTI resistance-inducing mutations and may have direct virucidal activity.
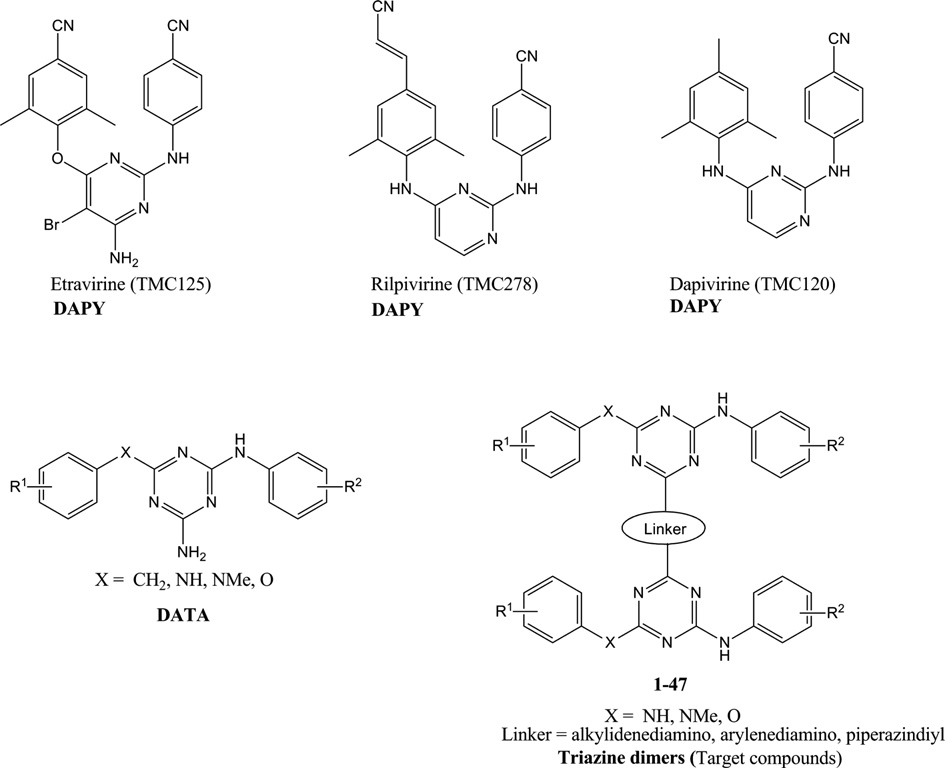
Chemical structures of DAPY, DATA and target compounds.
CAS number: 2450-71-7
Propargylamine is a colorless, odorless liquid that is known for its unique combination of an alkyne (triple bond) and an amine group, making it a versatile building block in organic synthesis and a valuable component in medicinal chemistry.
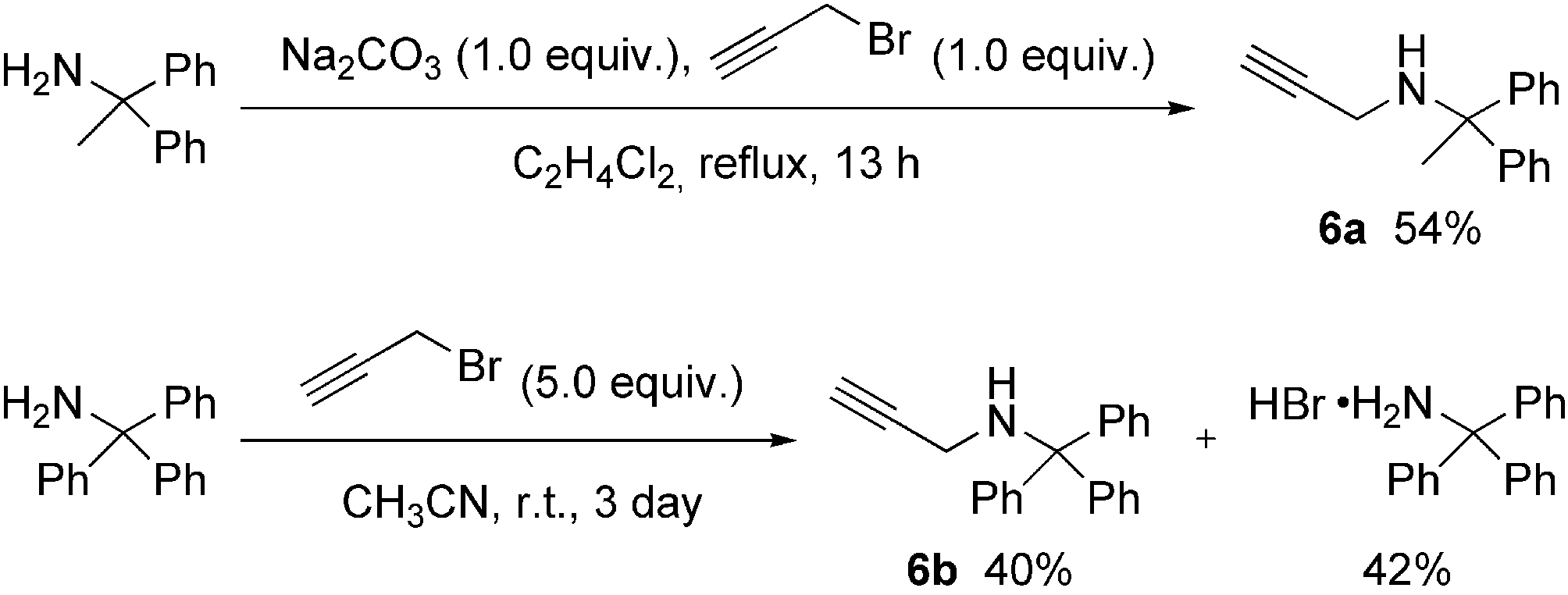
Preparation of propargylamines 6.
CAS number: 24517-64-4
2-Amino-3-cyanopyridine is known for its reactivity and use as an intermediate in the synthesis of various heterocyclic compounds. It features a pyridine ring with an amino group at the 2-position and a cyano group at the 3-position.
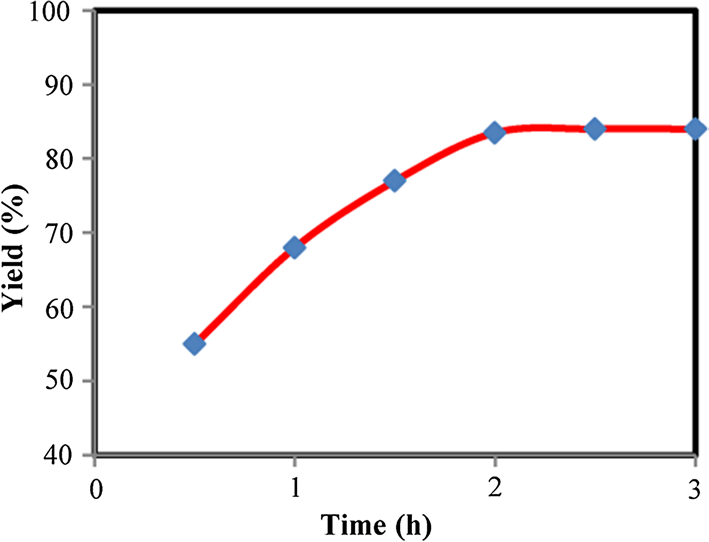
Effect of reaction time on the preparation of 2-amino-3-cyano-pyridine 3a.
CAS number: 24544-04-5
Benzenamine, 2,6-bis(1-methylethyl)- is a chemical compound also known as 2,6-Diisopropylaniline. It is an aromatic amine with two isopropyl groups attached to the benzene ring at positions 2 and 6.
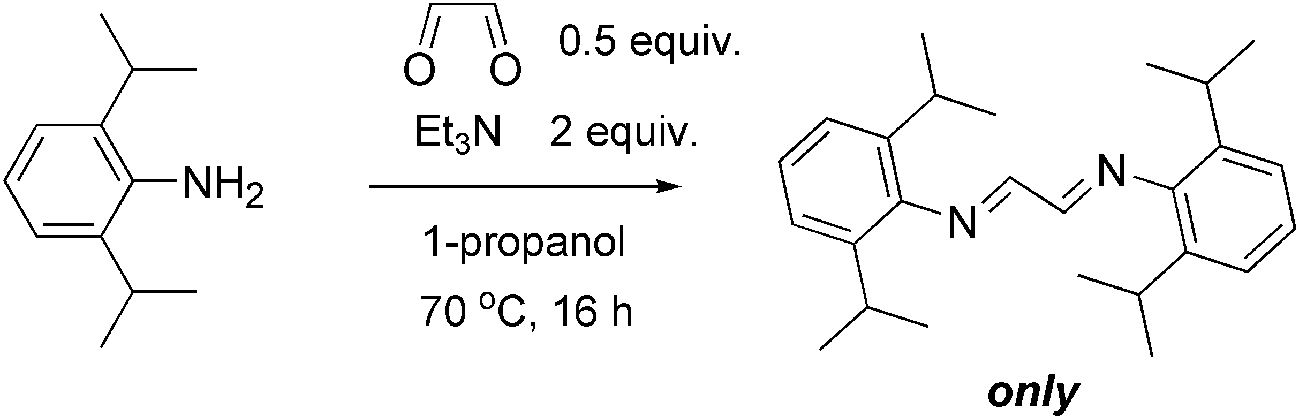
The reaction of glyoxal with 2,6-diisopropylaniline in the presence of triethylamine.
CAS number: 2465-56-7
Methanediyl is a carbene.
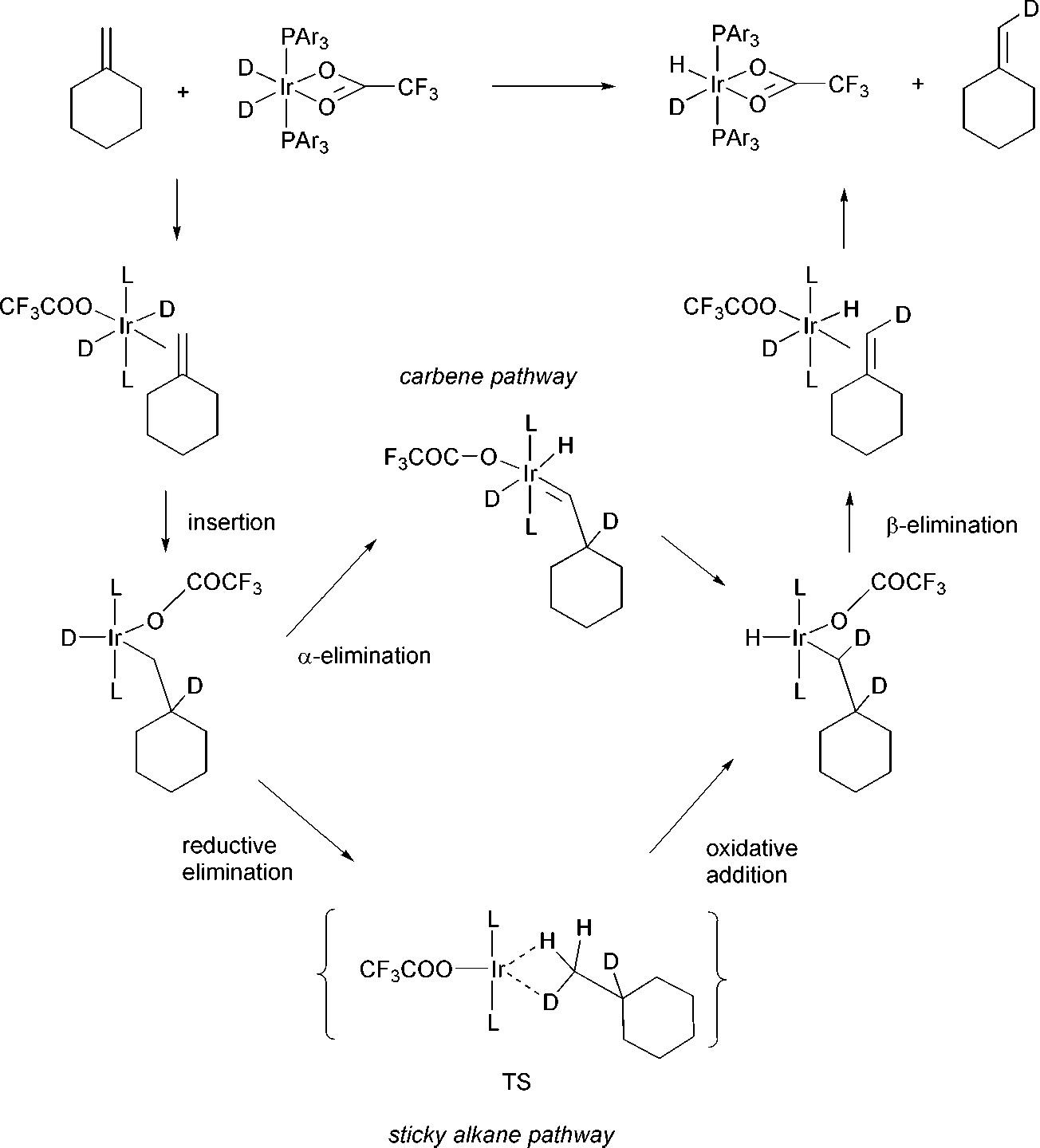
The mechanism for H/D exchange in methylenecyclohexane via the carbene and the alkane routes.
CAS number: 24949-00-6
Mukonidine is a member of carbazoles.
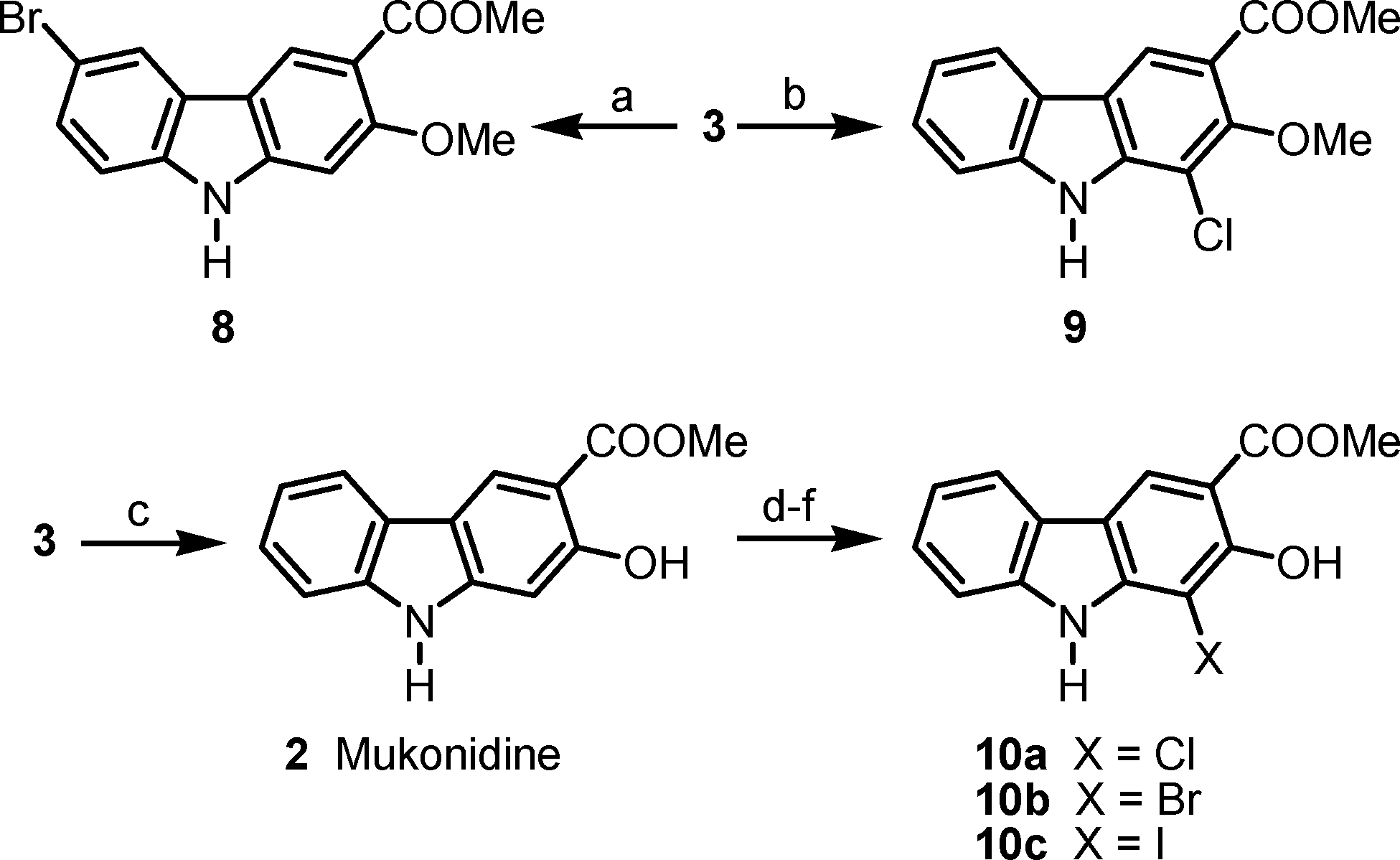
Halogenations of clausine L (3) and mukonidine (2).