Chemicals list & Research Gallery
CAS number: 21987-62-2
Bromophene (MC21-A) is a member of biphenyls and an organobromine compound. It has a role as a metabolite.
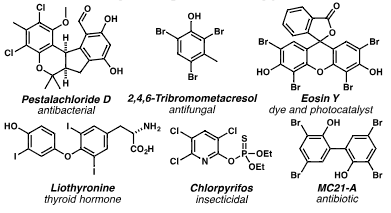
Selected haloarenes: Pestalachloride D, 2,4,6-Tribromo-m-cresol, Eosine Y, Liothyronine, Chlorpyrifos, mC21-A.
CAS number: 220127-57-1
Imatinib methanesulfonate, also known as STI-571, is a methanesulfonate (mesylate) salt that is the monomesylate salt of imatinib. Used for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours. It has a role as an antineoplastic agent, an apoptosis inducer, a tyrosine kinase inhibitor and an anticoronaviral agent. It contains an imatinib.
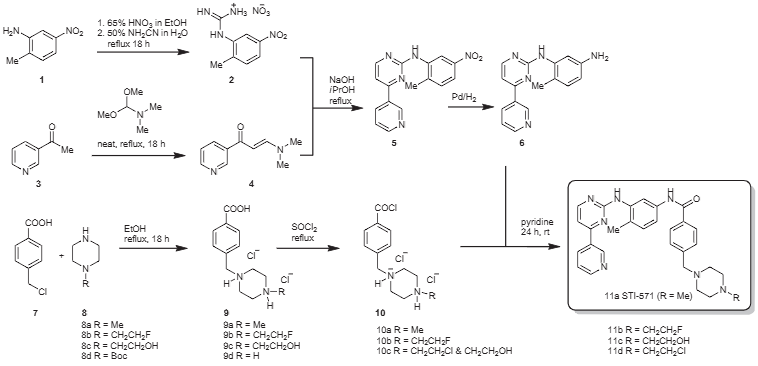
Synthesis of STI-571 and its analogs.
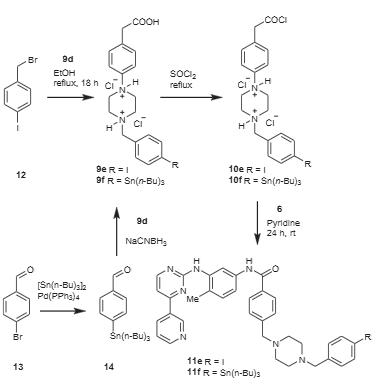
Synthesis of STI-I and STI-Sn analogs.
CAS number: 22025-87-2
2-tert-Butyl-N,N-dimethylaniline is an organic compound consisting of a benzene ring, a tert-butyl group at the 2-position, and a dimethylamino group (N,N-dimethyl) attached to the benzene ring. It is a tertiary aromatic amine.
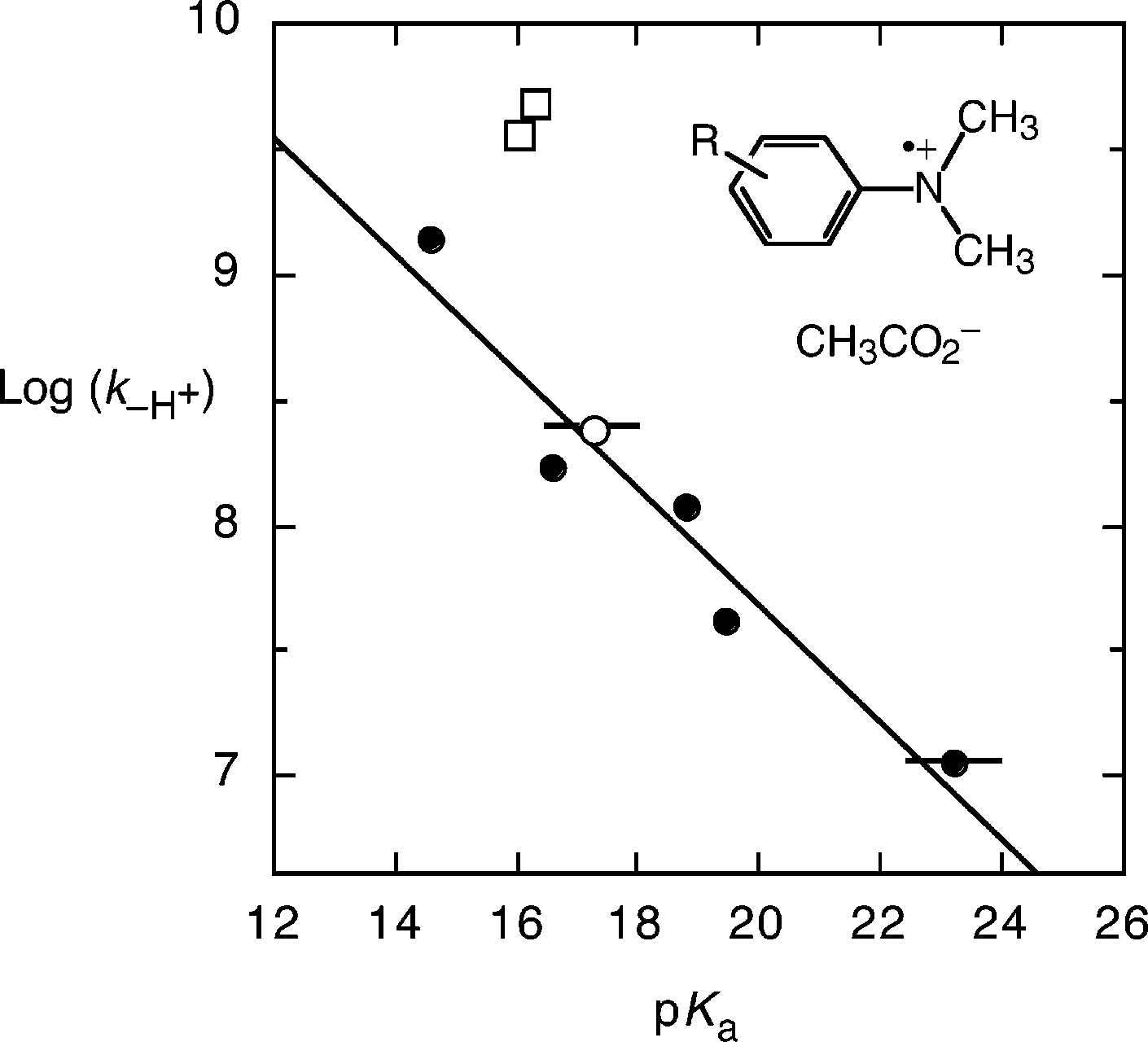
Brønsted plot of the logarithm of the bimolecular rate constant for deprotonation of the radical cations of N,N-dimethylaniline derivatives: (closed circles) 4-substituted-N,N-dimethylanilines, 1a-1e; (open circle) N,N,2-trimethylaniline, 2; (open squares) 2-tert-butyl-N,N-dimethylaniline, 3; and N,N,2,6-tetramethylaniline, 4.
CAS number: 22042-73-5
4-(2-Hydroxyethoxy)benzaldehyde, also known as Benzaldehyde, 4-(2-hydroxyethoxy)-, is an aromatic aldehyde featuring a benzene ring substituted at the para position with a 2-hydroxyethoxy group (–OCH₂CH₂OH) and bearing an aldehyde group (–CHO) directly attached to the ring. Its molecular formula is C₉H₁₀O₃, and it appears as a pale yellow to colorless crystalline solid or liquid with a mild, sweet, and slightly floral odor, reminiscent of other benzaldehyde derivatives. The presence of both hydroxyl and aldehyde functionalities gives it interesting reactivity, enabling it to participate in condensation, acetal formation, and esterification reactions, while the ether linkage contributes to its solubility in polar organic solvents.
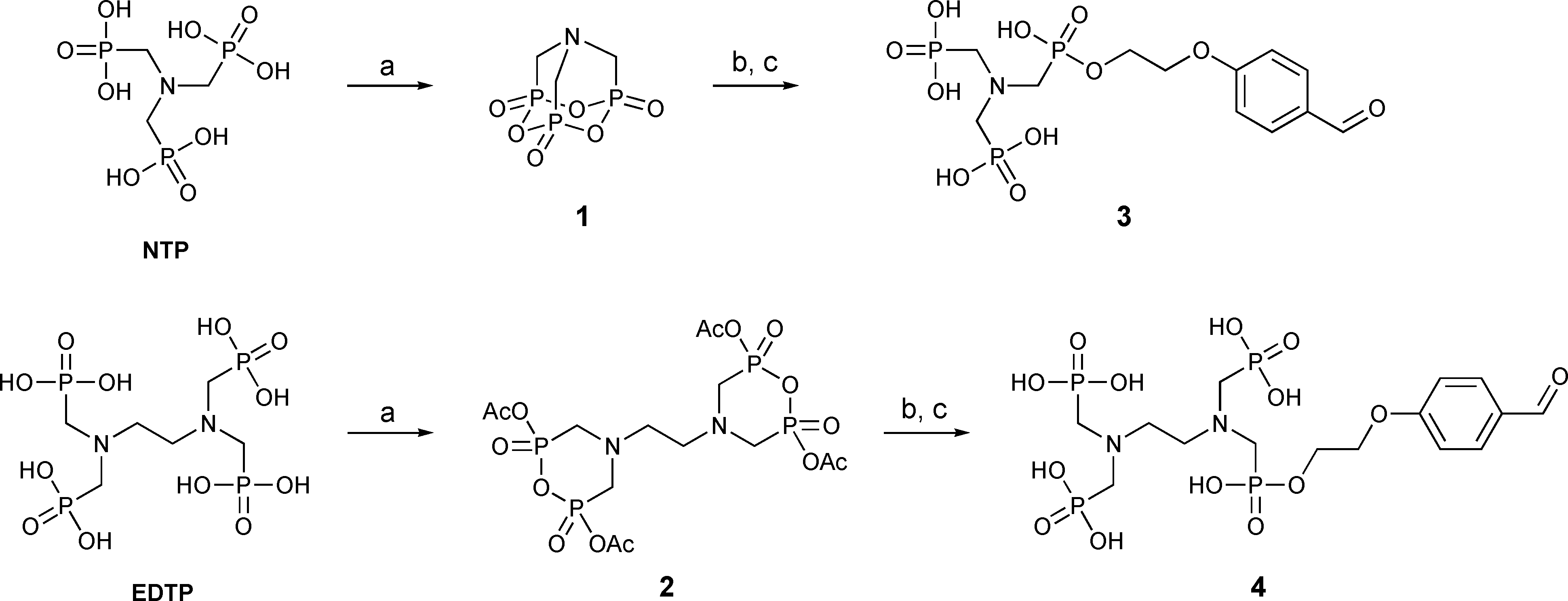
Preparation of the multiphosphonate building blocks 3 and 4. Reagents and conditions: a) acetic anhydride, DMF, b) 4-(2-hydroxyethoxy)-benzaldehyde, DMSO, c) water, DMSO.
CAS number: 22044-58-2
3-(1,3-benzodioxol-5-yl)-5,7-dihydroxy-6-(3-methylbut-2-enyl)-1-benzopyran-4-one is a member of isoflavanones.
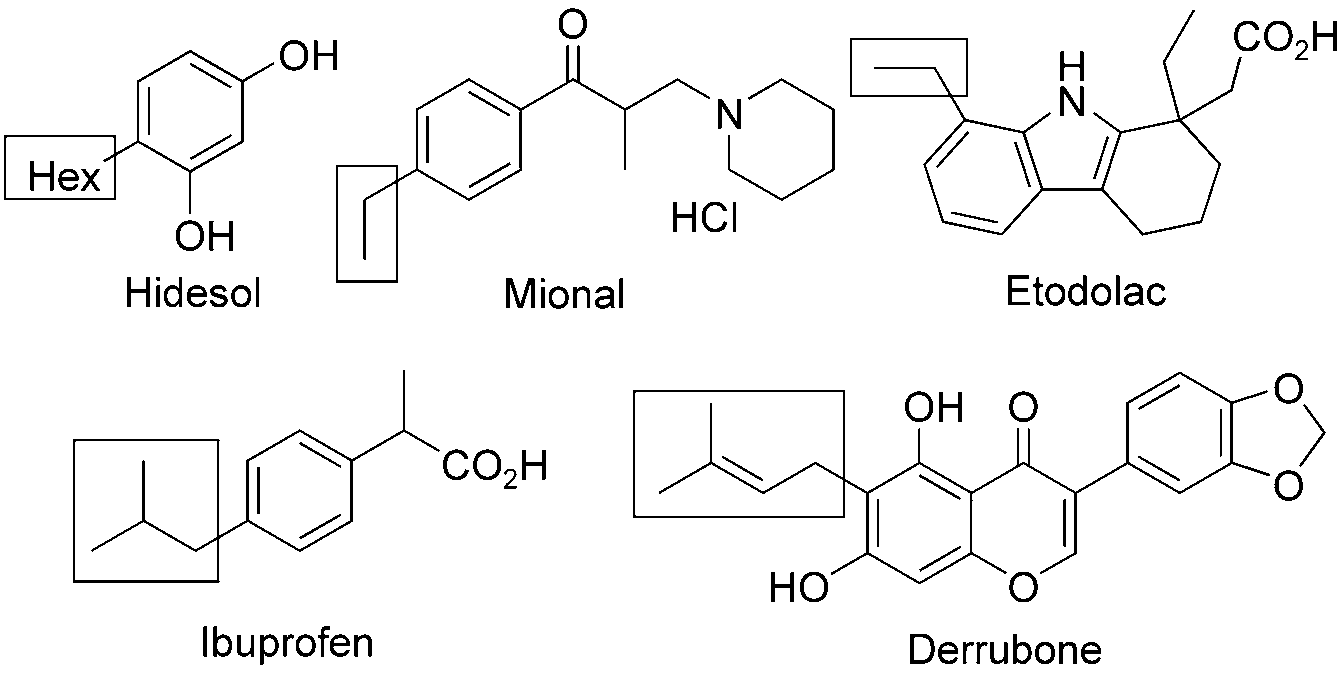
Drugs and bioactive natural products with alkyl side-chains: Hidesol, Mional, Etodolac, Derrubone
CAS number: 22052-71-7
8-Azachromone is a synthetic organic compound with the chemical name 4H-pyrano[2,3-b]pyridin-4-one. It is an aza-analogue of chromone, a natural product that is widely used in medicinal chemistry. The molecule is defined by a pyrone ring fused with a pyridine ring at the 2,3 position.
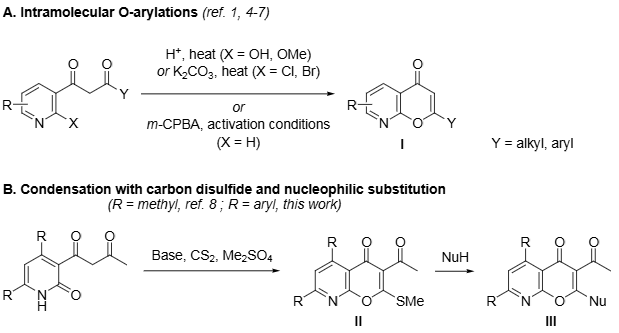
Synthesis of 8-Azachromones from 1,3-Diketone Precursors.
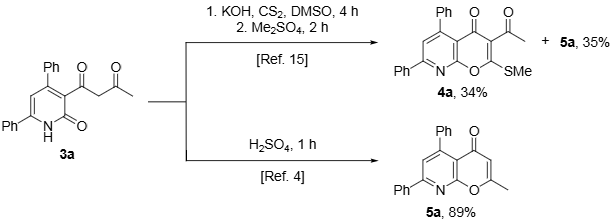
Cyclization of Diketone 3a into 8-Azachromones According to Reported Procedures.
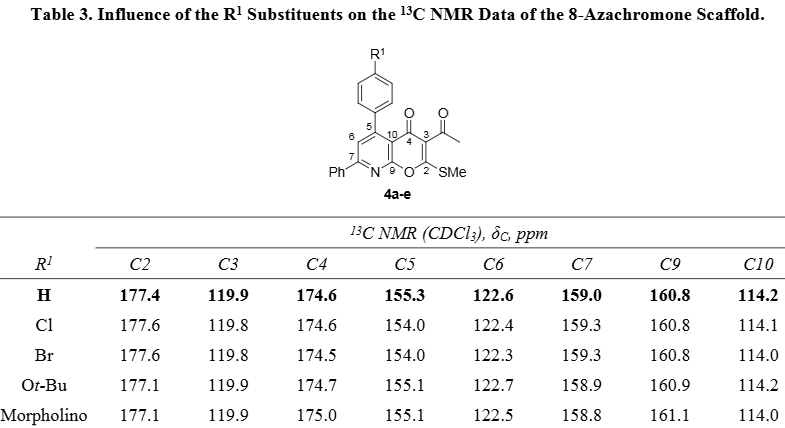
Influence of the R1 Substituents on the 13C NMR Data of the 8-Azachromone Scaffold.
CAS number: 22071-15-4
Ketoprofen, a propionic acid derivative, is a nonsteroidal anti-inflammatory agent (NSAIA) with analgesic and antipyretic properties.
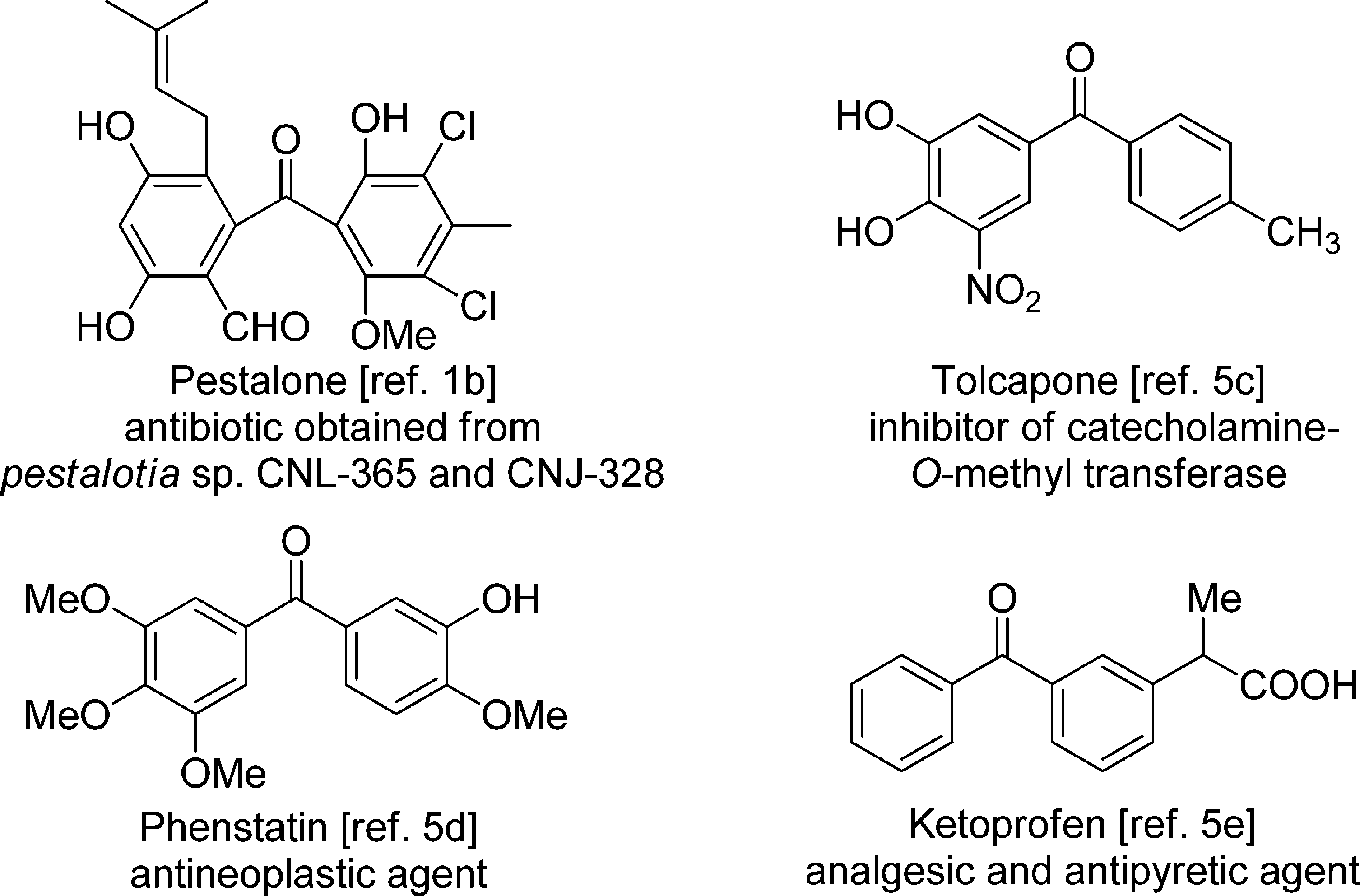
Bioactive Compounds with the Diarylmethanone Motif: Pestalone, Tolcapone, Phenstatin, Ketoprofen
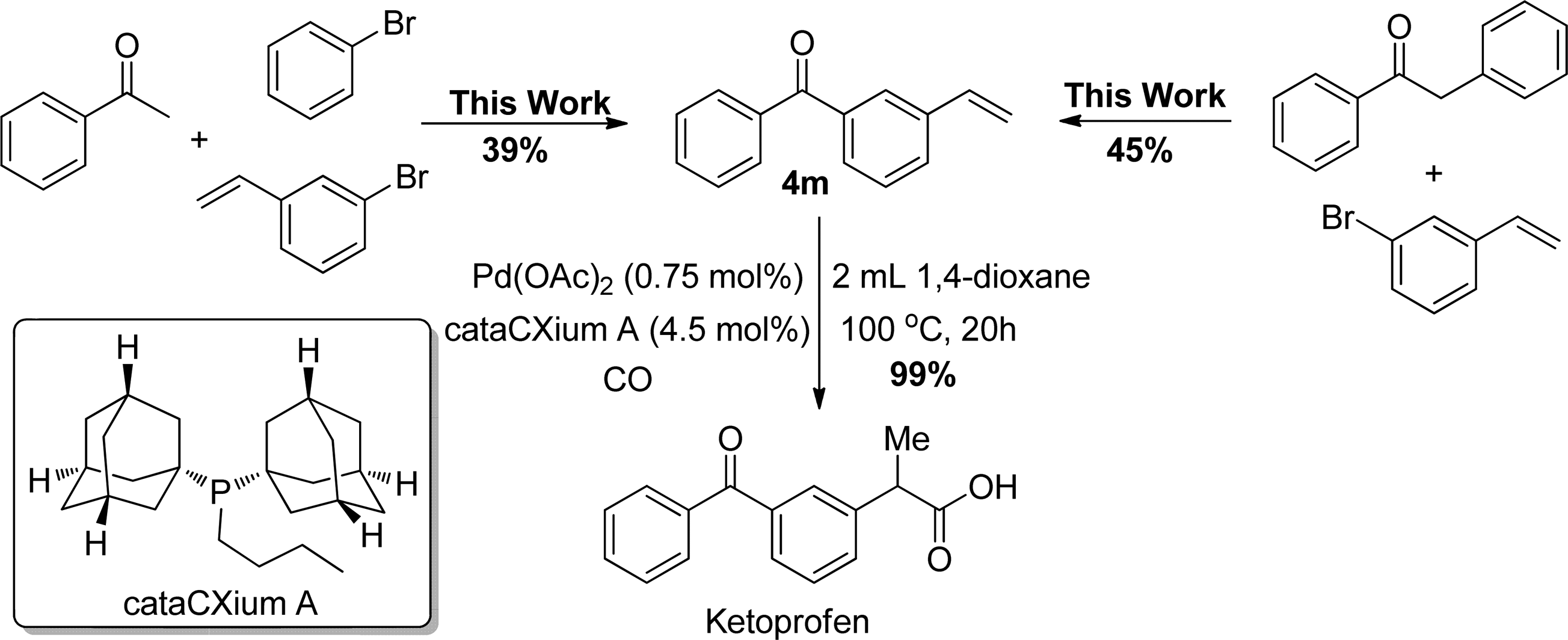
Synthetic Application of This Methodology to the Preparation of Ketoprofen
CAS number: 220728-17-6
UCL-1972 is a small-molecule, non-imidazole histamine H₃ receptor antagonist originally developed at University College London, under investigation for potential use in cognitive disorders.
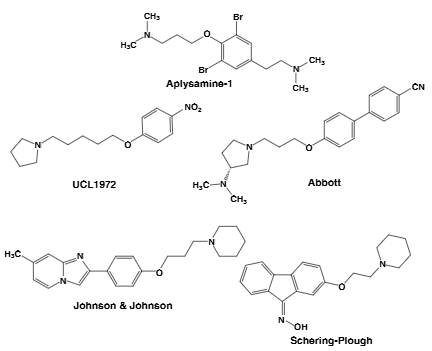
Examples of non-imidazole-derived H3 antagonists: Aplysamine-1, UCL-1972, Abbott(A-10749).
CAS number: 2216-94-6
Ethyl phenylpropiolate is a clear pale yellow liquid.
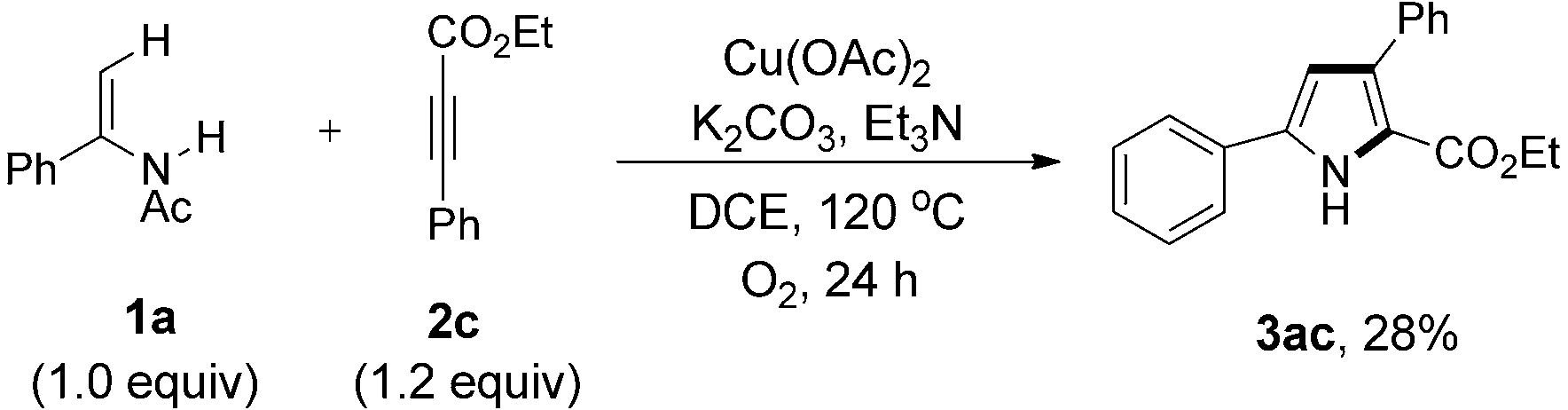
Coupling reaction of enamide 1a with ethyl 3-phenylpropiolate 2c.
CAS number: 22204-53-1
Naproxen is classified as a nonsteroidal anti-inflammatory dug (NSAID) and was initially approved for prescription use in 1976 and then for over-the-counter (OTC) use in 1994. It can effectively manage acute pain as well as pain related to rheumatic diseases, and has a well studied adverse effect profile. Given its overall tolerability and effectiveness, naproxen can be considered a first line treatment for a variety of clinical situations requiring analgesia. Naproxen is available in both immediate and delayed release formulations, in combination with sumatriptan to treat migraines, and in combination with esomeprazole to lower the risk of developing gastric ulcers.
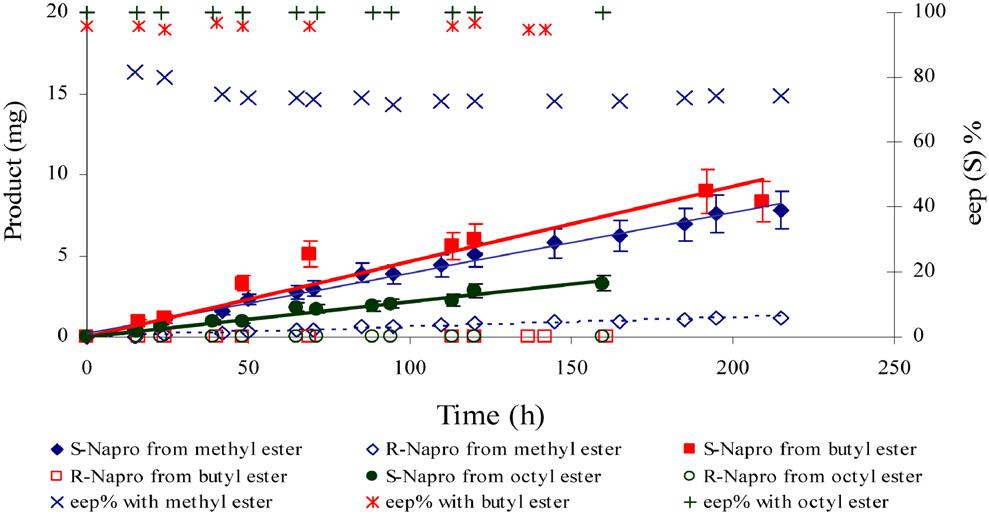
Behavior of (S)- and (R)-naproxen acid and enantiomeric excess of immobilized lipase in the two-separate-phase membrane reactor during the hydrolysis of naproxen esters.