Chemicals list & Research Gallery
CAS number: 16722-51-3
Toluene-4-sulfonate is an arenesulfonate oxoanion that is the conjugate base of toluene-4-sulfonic acid. It is a conjugate base of a toluene-4-sulfonic acid.
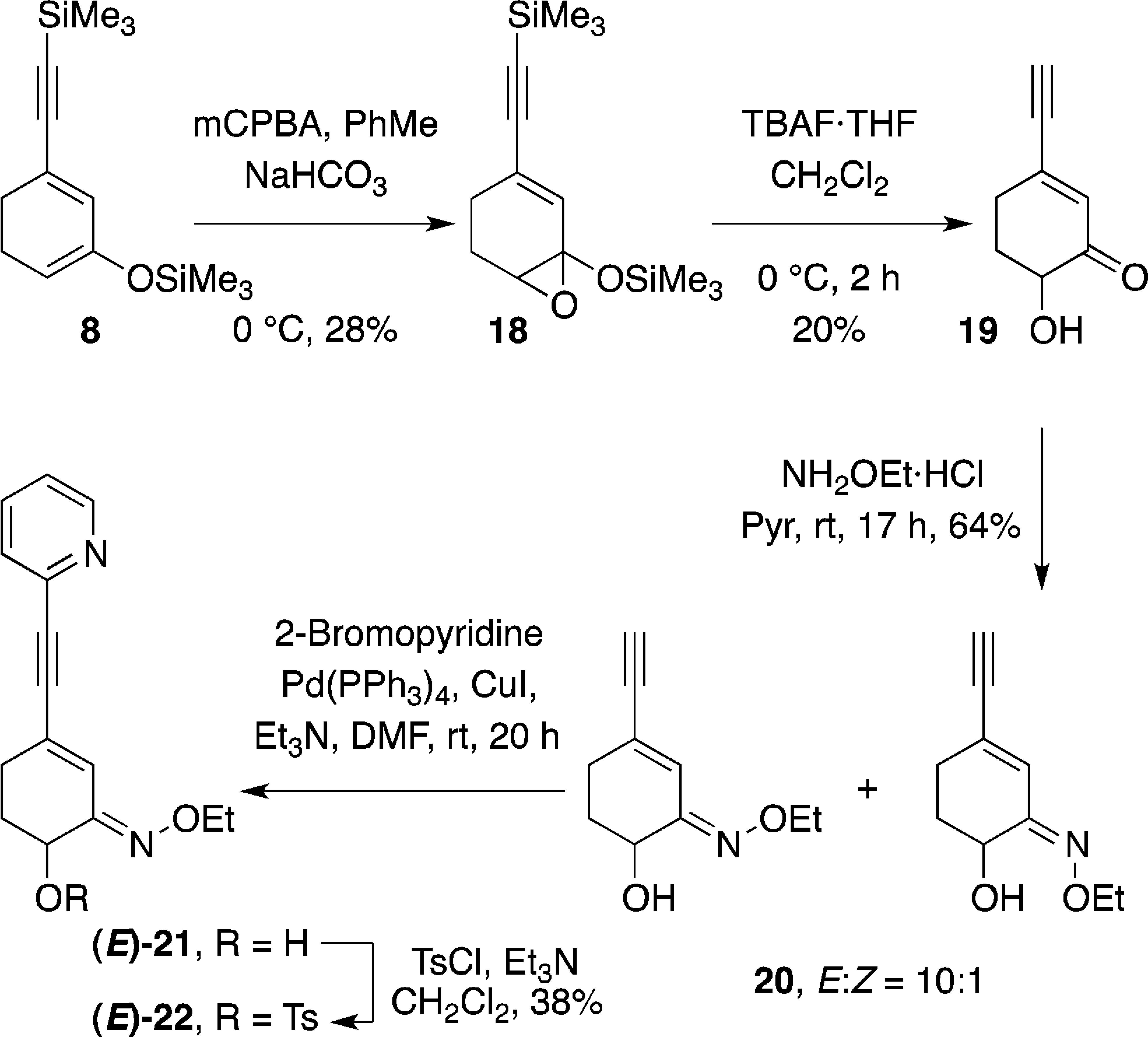
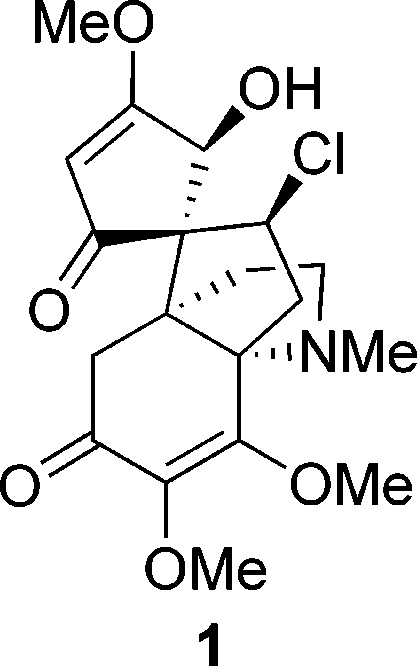
Synthesis of Radiolabelling Precursor Tosylate (E)-22 via the Rubottom Oxidation
CAS number: 16753-36-9
Copper(I) acetylide (Cu2C2) is a reddish, explosive solid, produced by passing acetylene through copper(I) chloride solution.
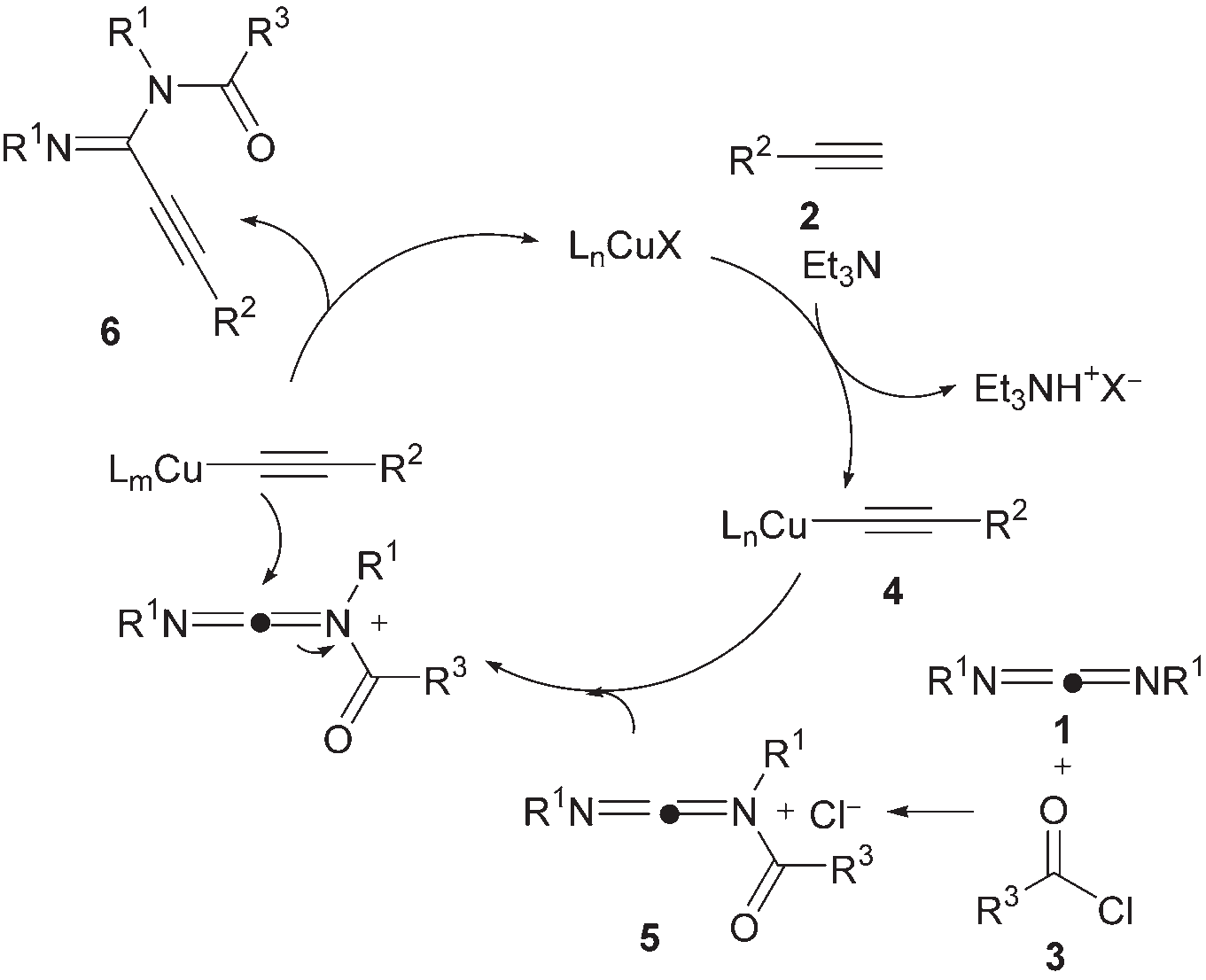
Carbodiimide 1 reacts with acyl chloride 3 to generate N-acylimide salt 5. Alkyne 2 is immediately converted to acetylide copper 4 in the presence of triethylamine, and 1 equivalent of triethylamine acid salt is released. Subsequently, 4 undergoes nucleophilic attack on 5 to obtain the target product 6, and the copper catalyst is released, completing the catalytic cycle.
CAS number: 1689-71-0
Cis-stilbene oxide is a stilbene oxide and an epoxide.
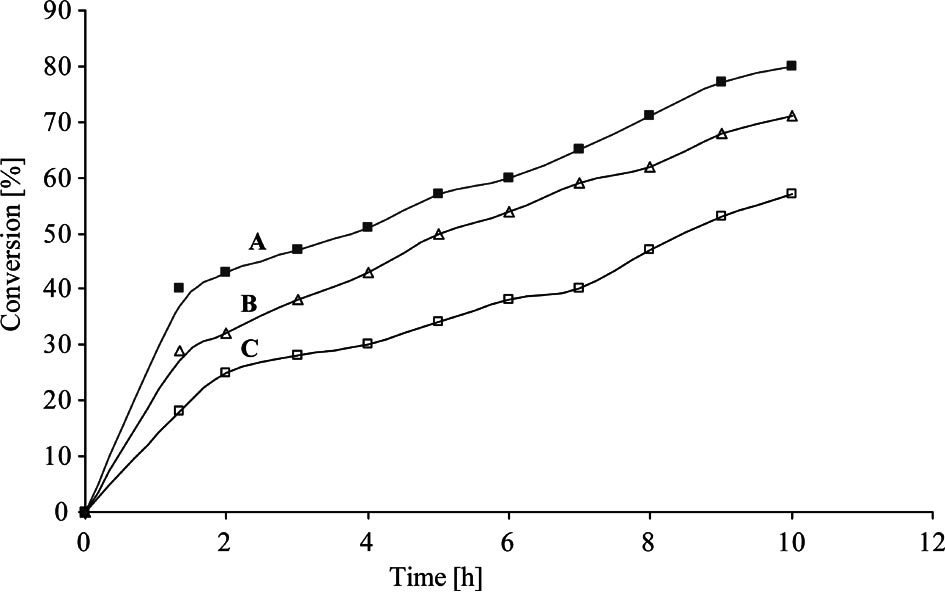
% Conversion of syn-β-amino alcohol obtained from ring opening of meso-stilbene oxide with aniline vs. time for different M/L ratios.
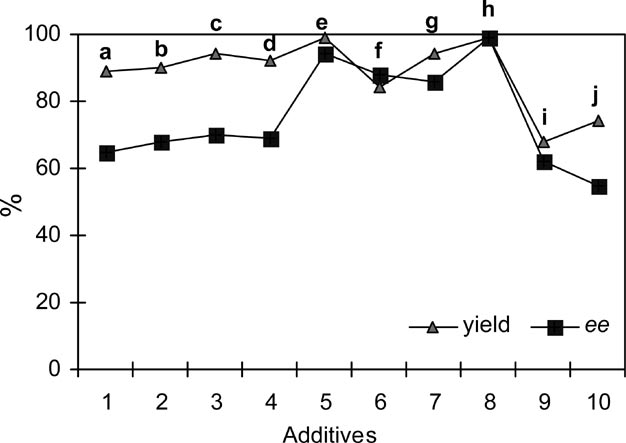
% Yields and ee values (%) for ring opening reactions of meso-stilbene oxide with aniline in the presence of different additives in 10 h.
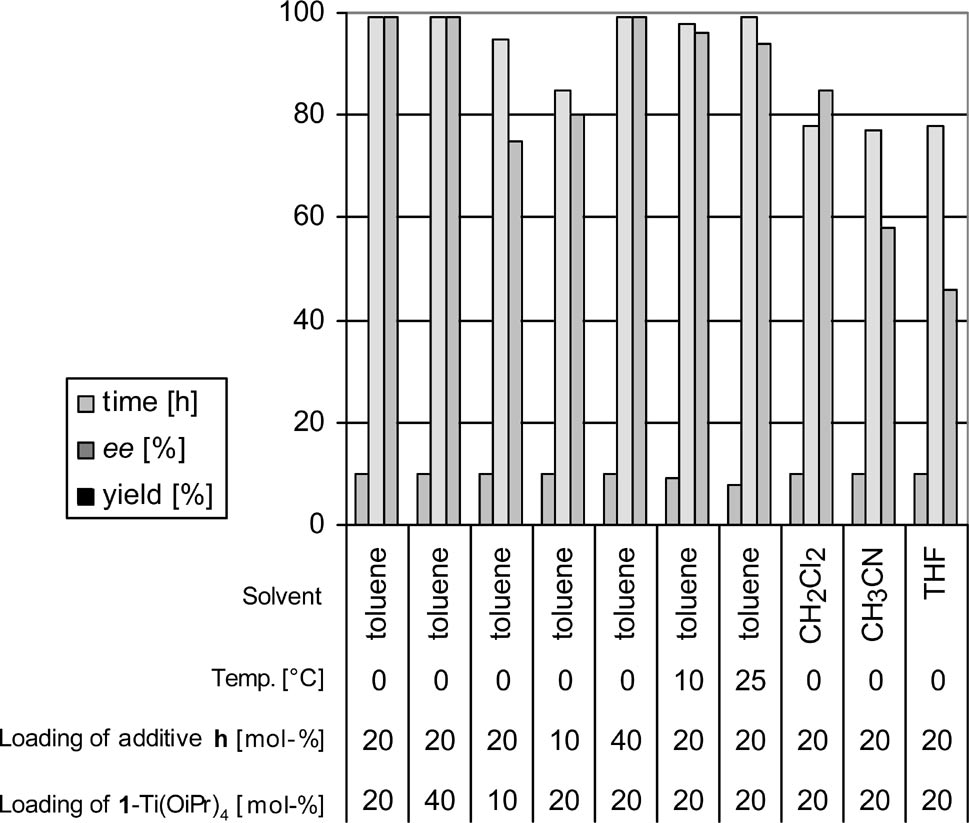
Optimization of reaction conditions for ring opening of meso-stilbene oxide with aniline in the presence of 2-MeO imine (h) as additive.
CAS number: 16940-66-2
Sodium borohydride is a low-cost inorganic compound with unique reduction, bleaching, brightening, and purification properties.
![No Reaction of Bromoacetal (1Br) in the Presence of [HFeCl(dppe)2] (3) and NaBH4](http://www.wlxkc.cn/picture/947257_06.png)
No Reaction of Bromoacetal (1Br) in the Presence of [HFeCl(dppe)2] (3) and NaBH4
CAS number: 169590-42-5
Celecoxib is a member of the class of pyrazoles that is 1H-pyrazole which is substituted at positions 1, 3 and 5 by 4-sulfamoylphenyl, trifluoromethyl and p-tolyl groups, respectively. A cyclooxygenase-2 inhibitor, it is used in the treatment of arthritis. It has a role as a cyclooxygenase 2 inhibitor, a geroprotector, a non-steroidal anti-inflammatory drug and a non-narcotic analgesic. It is a member of toluenes, a sulfonamide, a member of pyrazoles and an organofluorine compound.
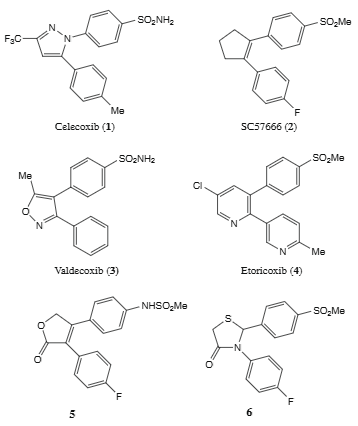
Representative examples of selective COX-2 inhibitors: Celecoxib, SC57666, Valdecoxib, Etoricoxib.
CAS number: 17088-37-8
Triacetone triperoxide (TATP) is a high-power explosive which is often used by criminals.
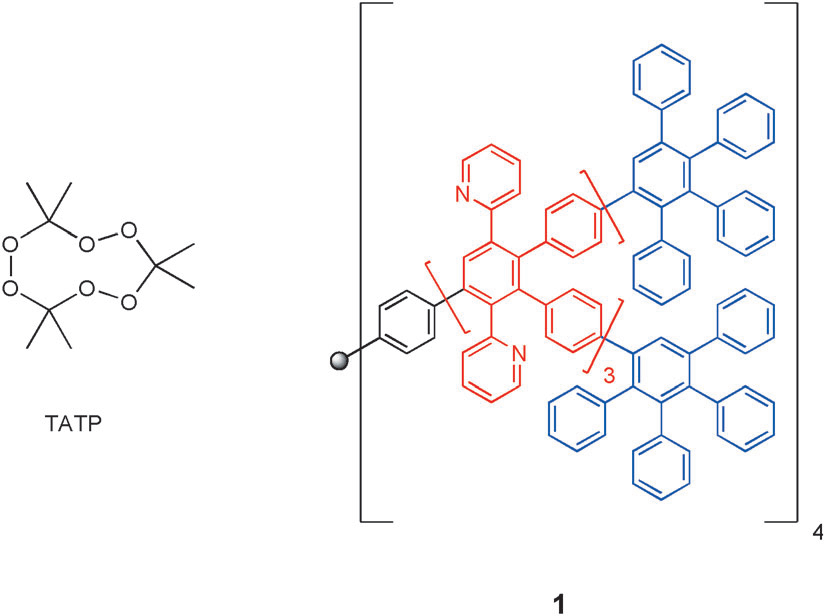
Structure of triacetone triperoxide (TATP) and the reference dendrimer 1.
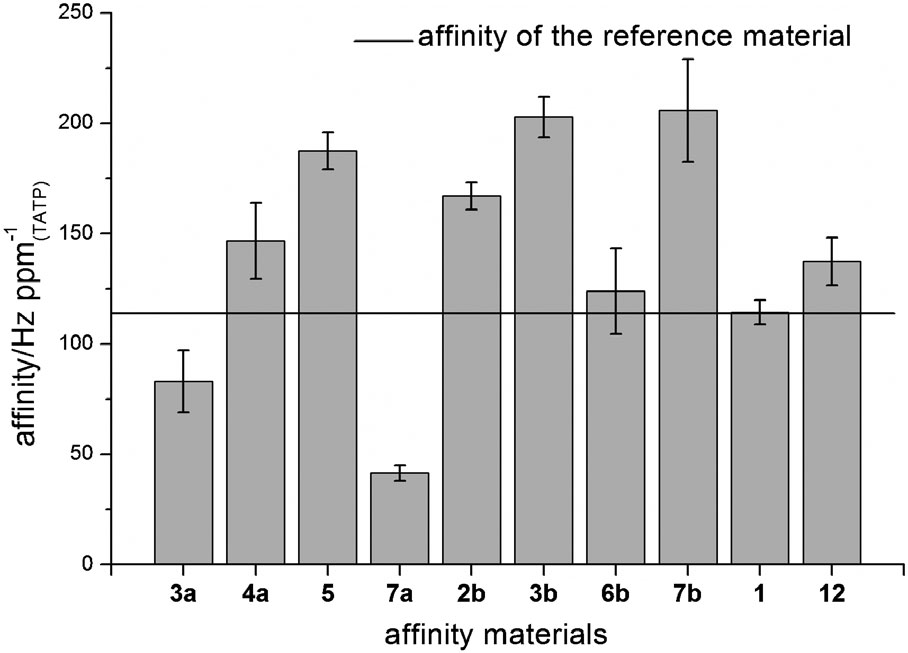
Slopes of adsorption isotherms for TATP. Best known system poly- phenylen-pyridyl dendrimer 1 is indicated by the horizontal line.
CAS number: 17101-36-9
Diphosphite, also known as pyrophosphite, refers to a chemical compound or salt containing two phosphite groups or the anion P2O54-. It can be produced by gently heating acid phosphites under reduced pressure. In the context of chemistry, it can also refer to a chemical containing two phosphite groups.
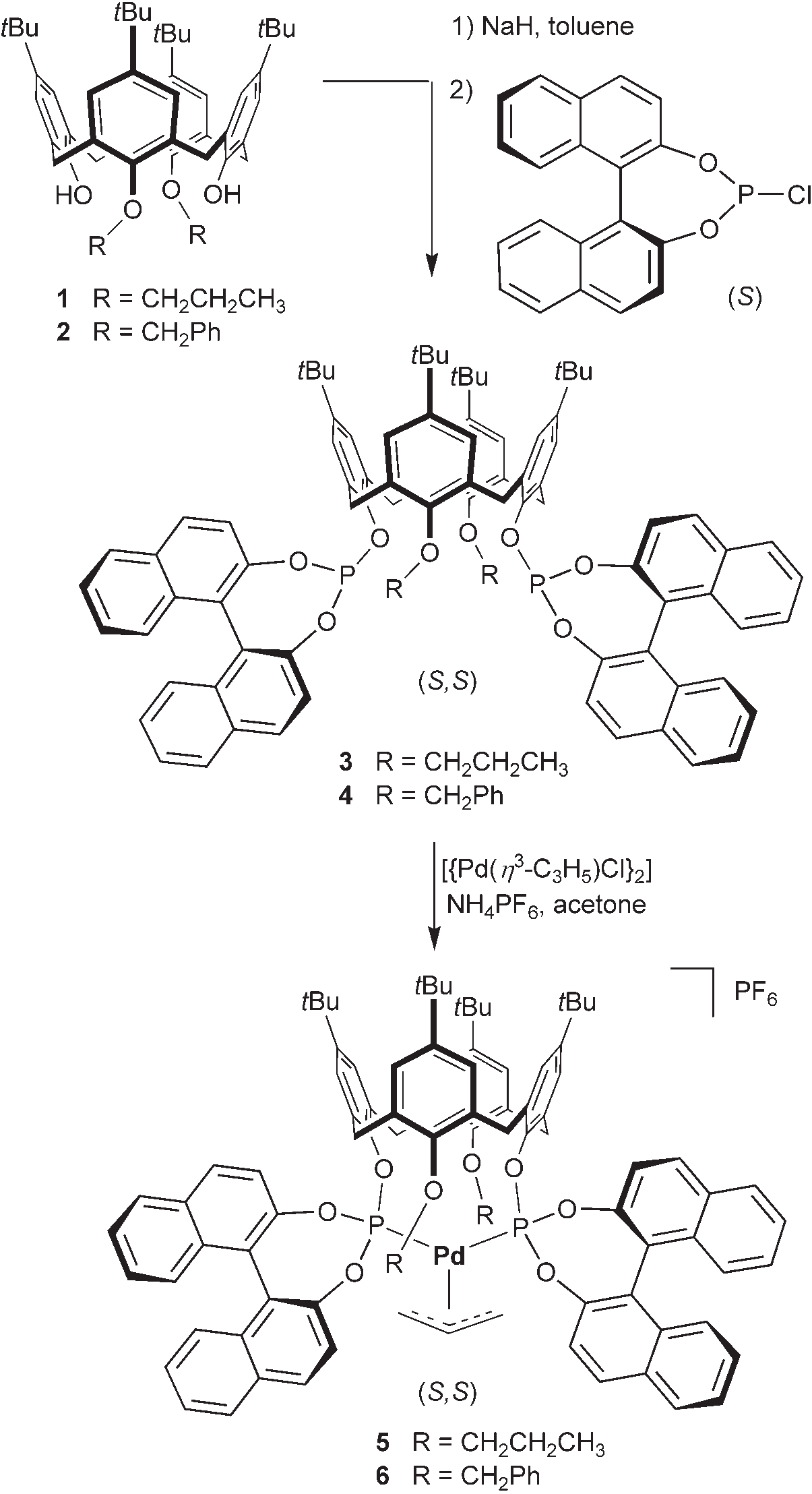
Synthesis of the diphosphites and their cationic allyl–palladium complexes.
CAS number: 171864-80-5
Tryprostatin A is a cyclic dipeptide that is brevianamide F (cyclo-L-Trp-L-Pro) substituted at positions 2 and 6 on the indole ring by prenyl and methoxy groups respectively. It has a role as a breast cancer resistance protein inhibitor. It is a dipeptide, a member of indoles, a pyrrolopyrazine, an aromatic ether and an indole alkaloid. It is functionally related to a brevianamide F.
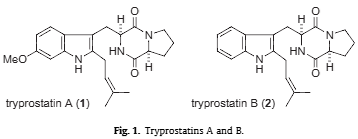
Tryprostatins A and B.
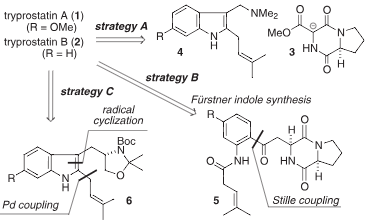
Retrosynthetic analysis for tryprostatins A and B.
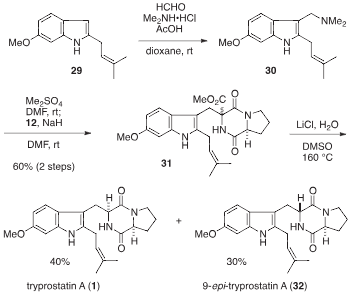
Total synthesis of tryprostatin A (1).
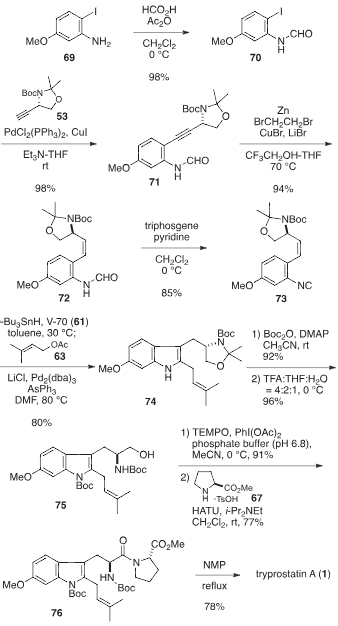
Total synthesis of tryprostatin A.
CAS number: 172678-62-5
HMR 3004 (also known as RU 64004) is a chemical compound classified as a ketolide. Ketolides are a novel class of semisynthetic 14-membered-ring macrolide antibiotics derived from erythromycin A. The distinguishing feature of ketolides like HMR 3004 is the presence of a 3-keto group on the erythronolide A ring instead of the l-cladinose sugar found in traditional macrolides like erythromycin.
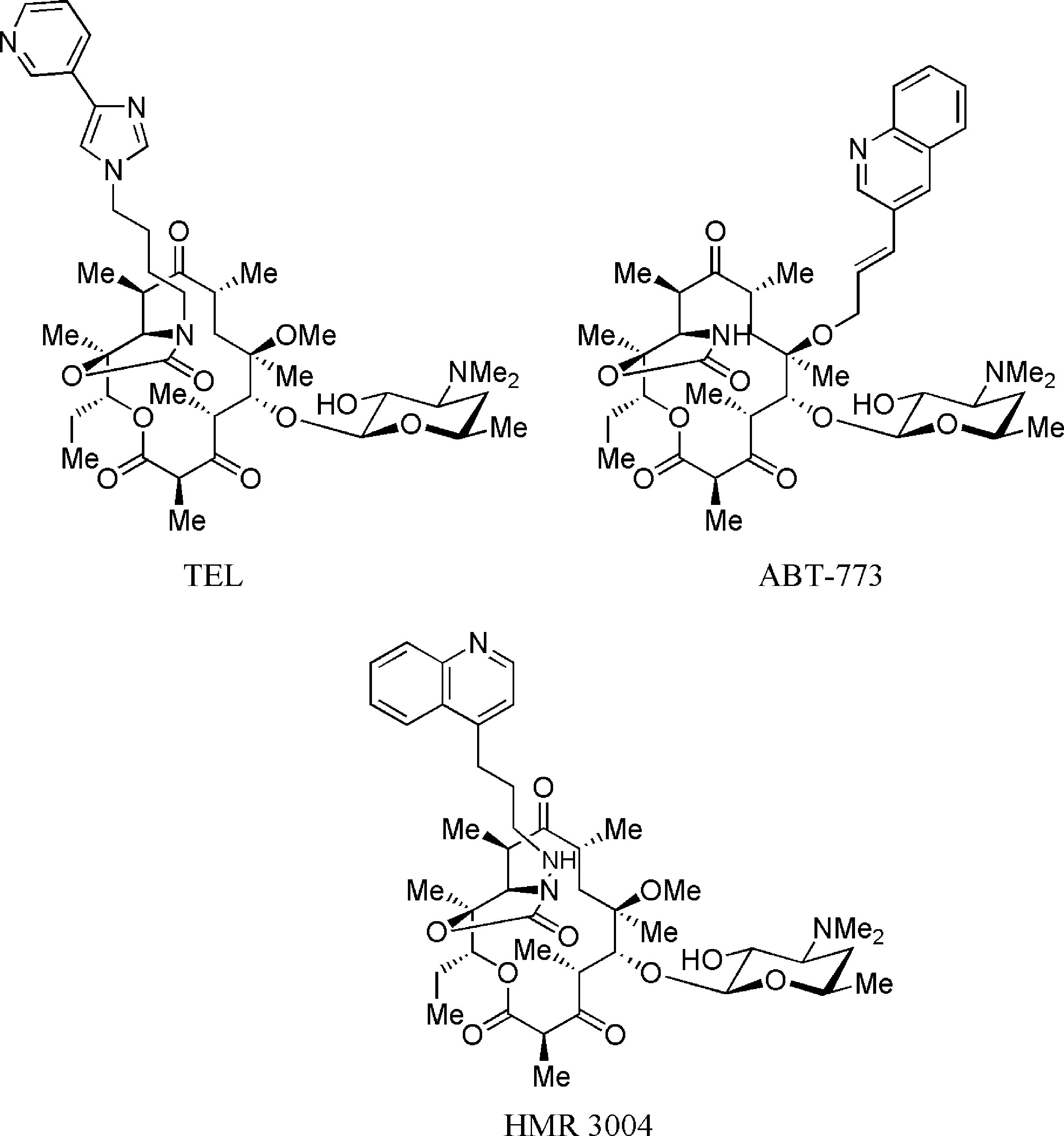
Structures of ketolides: Telithromycin, ABT-773, Hmr 3004.