Copper acetylide
CAS number: 16753-36-9
Copper(I) acetylide (Cu2C2) is a reddish, explosive solid, produced by passing acetylene through copper(I) chloride solution.
Related images
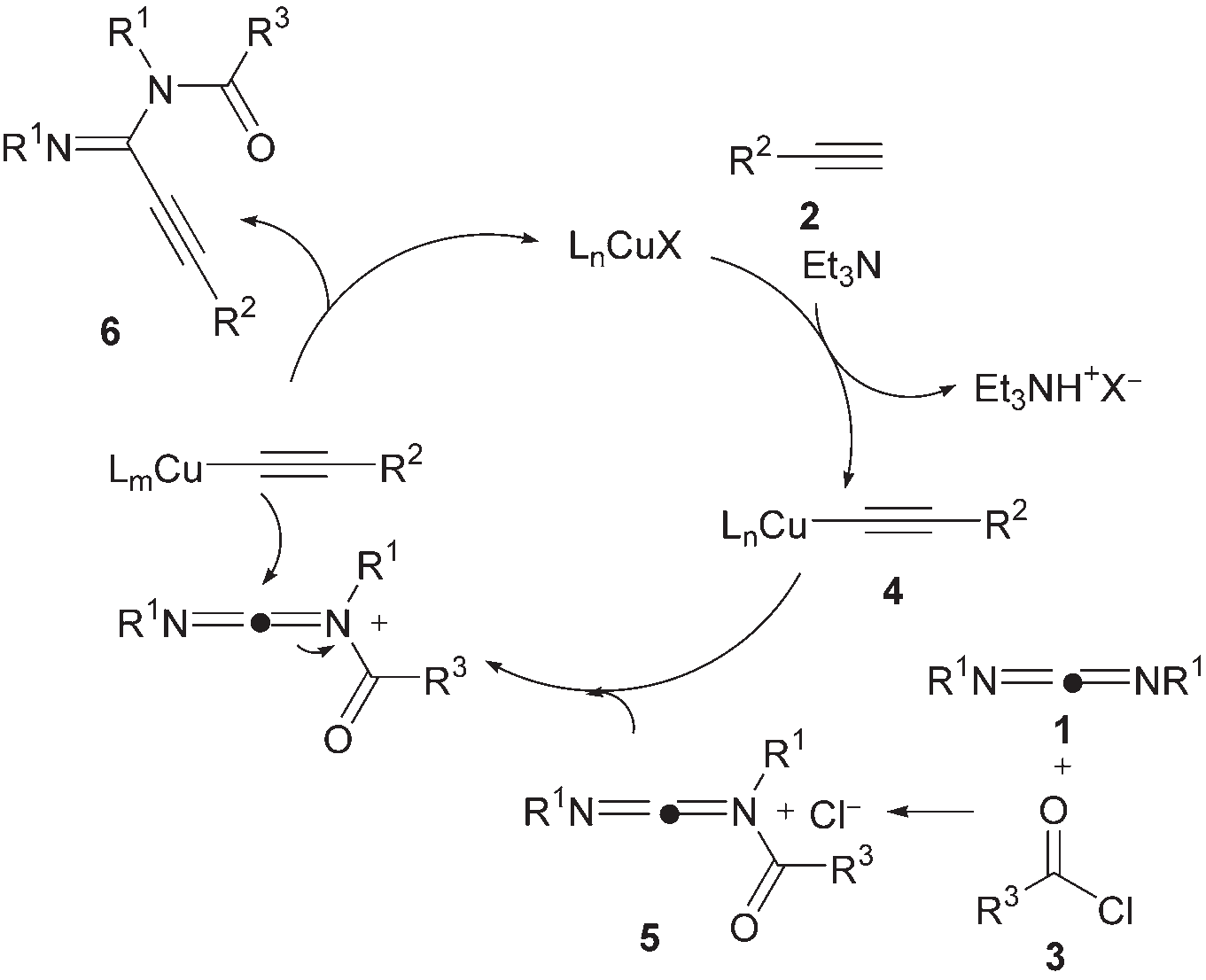
Carbodiimide 1 reacts with acyl chloride 3 to generate N-acylimide salt 5. Alkyne 2 is immediately converted to acetylide copper 4 in the presence of triethylamine, and 1 equivalent of triethylamine acid salt is released. Subsequently, 4 undergoes nucleophilic attack on 5 to obtain the target product 6, and the copper catalyst is released, completing the catalytic cycle.
Related Questions and Answers
No related questions yet