Carbodiimide
CAS number: 151-51-9
Carbodiimide is a commonly used water loss agent, mainly used to activate carboxyl groups to promote the formation of amides and esters.
Related images
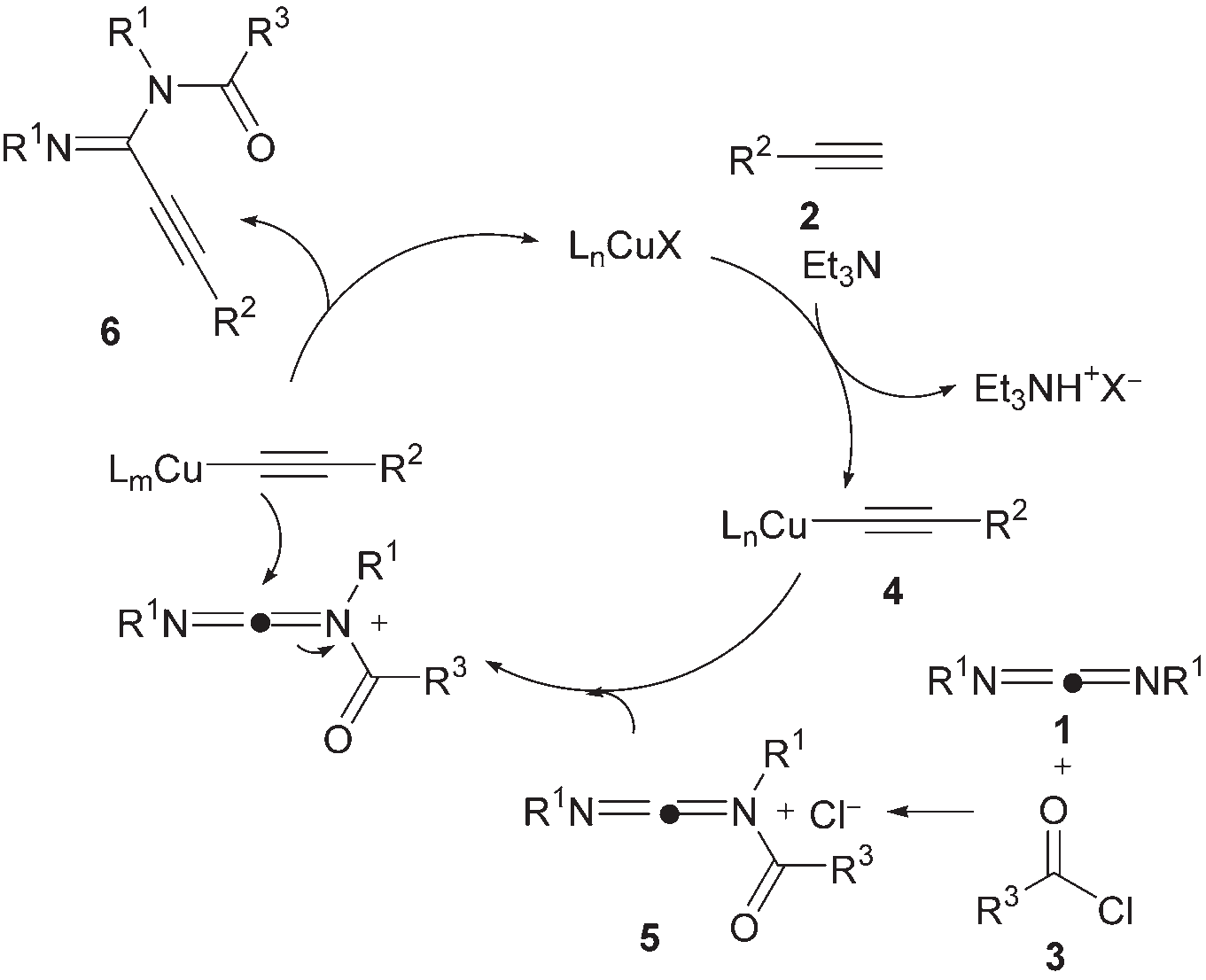
Carbodiimide 1 reacts with acyl chloride 3 to generate N-acylimide salt 5. Alkyne 2 is immediately converted to acetylide copper 4 in the presence of triethylamine, and 1 equivalent of triethylamine acid salt is released. Subsequently, 4 undergoes nucleophilic attack on 5 to obtain the target product 6, and the copper catalyst is released, completing the catalytic cycle.
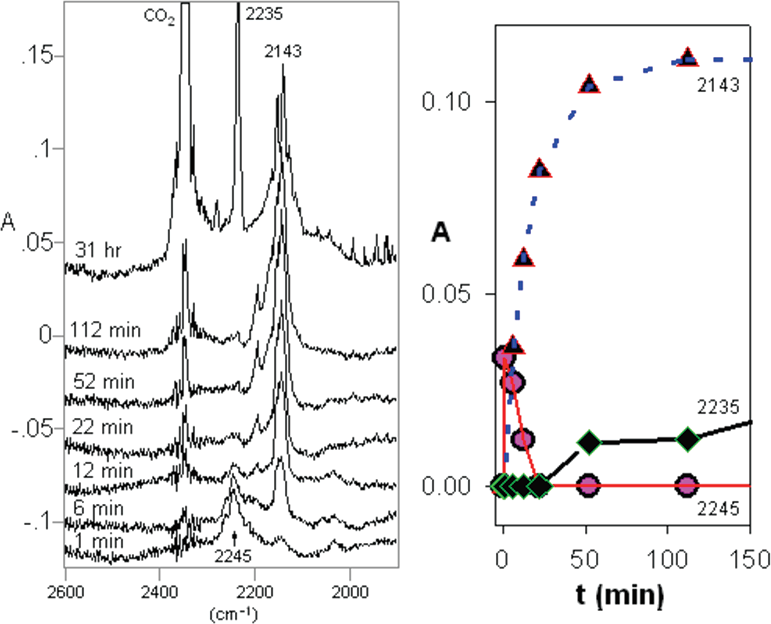
Partial IR difference spectra of 5-phenyl-2-(trimethylsilyl)-tetrazole 22 at 12 K in Ar matrix at different photolysis times at 254 nm, showing peaks due to photolysis products. Abscissa 1900−2600 cm−1. (Right) Plots of IR absorbance of different wavenumbers versus photolysis time from 0 to 150 min. The 2245 and 2143 cm−1 bands are assigned to nitrile imine 23 and carbodiimide 25, respectively, and the 2235 cm−1 band is assigned to benzonitrile.
Related Questions and Answers
No related questions yet