Chemicals list & Research Gallery
CAS number: 59-92-7
Levodopa is a prodrug of dopamine that is administered to patients with Parkinson's due to its ability to cross the blood-brain barrier. Levodopa can be metabolised to dopamine on either side of the blood-brain barrier and so it is generally administered with a dopa decarboxylase inhibitor like carbidopa to prevent metabolism until after it has crossed the blood-brain barrier. Once past the blood-brain barrier, levodopa is metabolized to dopamine and supplements the low endogenous levels of dopamine to treat symptoms of Parkinson's. The first developed drug product that was approved by the FDA was a levodopa and carbidopa combined product called Sinemet that was approved on May 2, 1975.
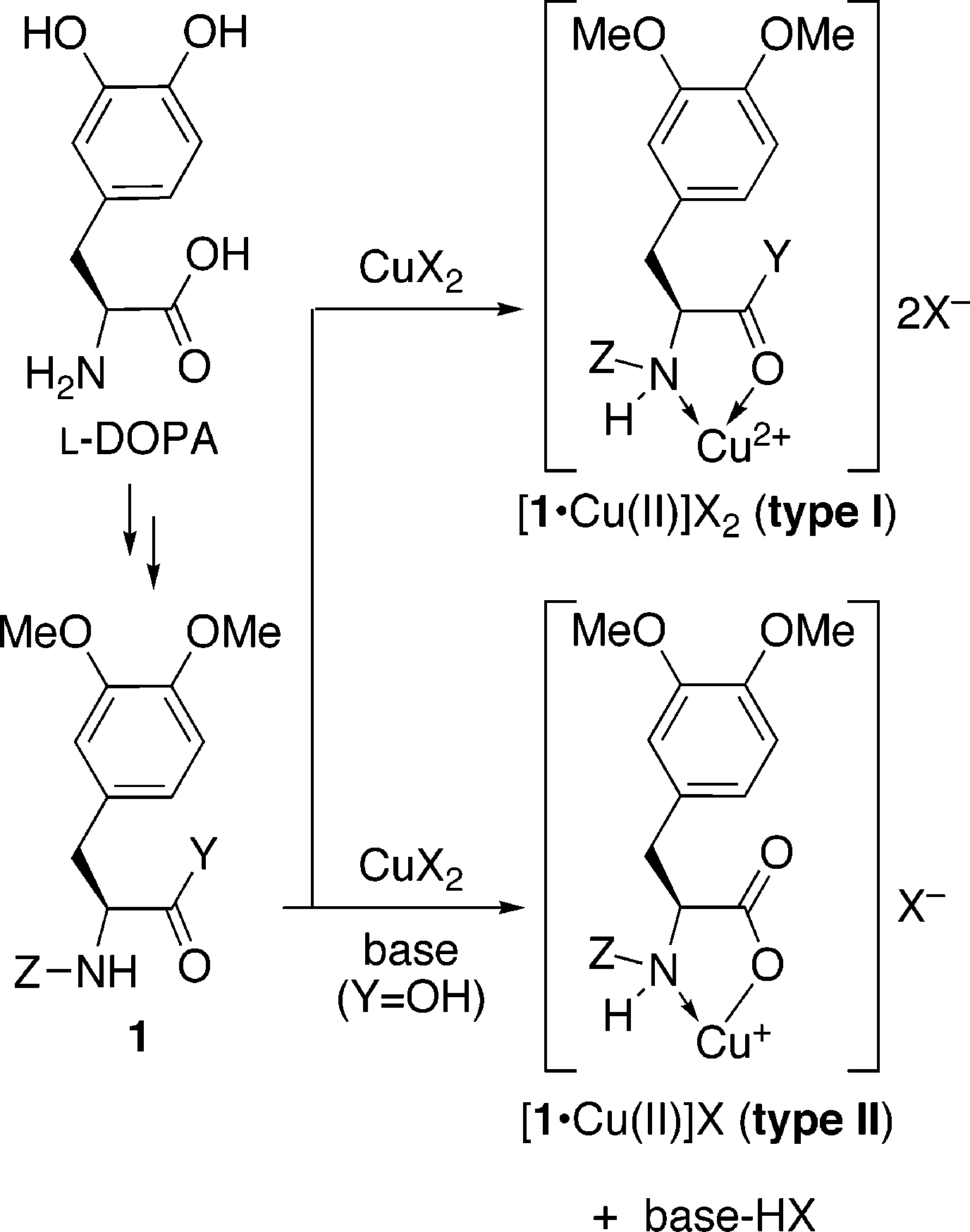
Design of l-DOPA Derivatives 1•Cu(II) Complexes
CAS number: 5906-30-9
1-Piperazinesulfonamide is a sulfonamide derivative of piperazine, featuring a six-membered piperazine ring with two nitrogen atoms at positions 1 and 4. The sulfonamide group (–SO2NH2) is attached to one of the nitrogen atoms, contributing to its potential biological activity, particularly as an enzyme inhibitor or antimicrobial agent.
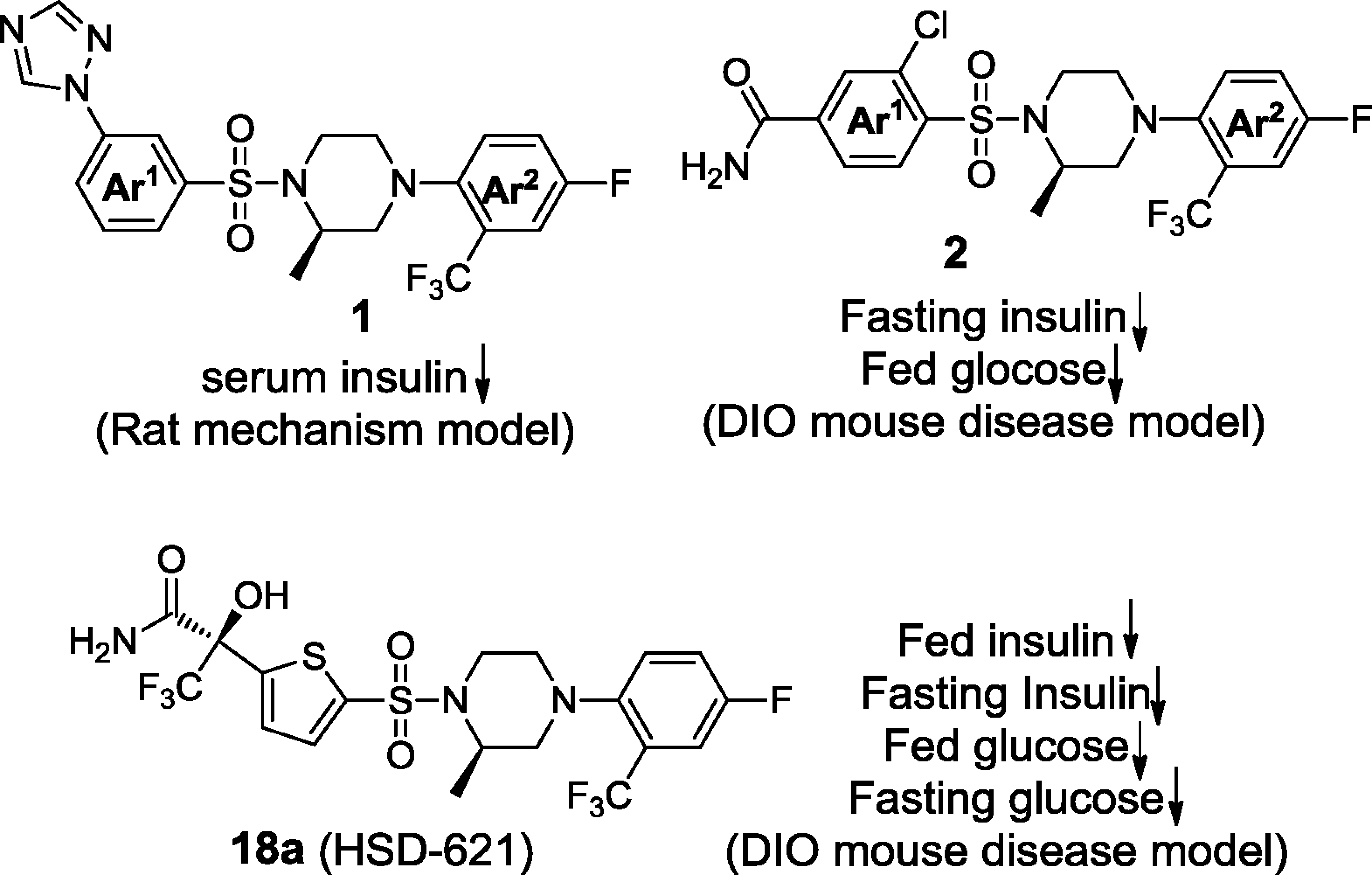
Potent and selective piperazine sulfonamides as 11β-HSD1 inhibitors.
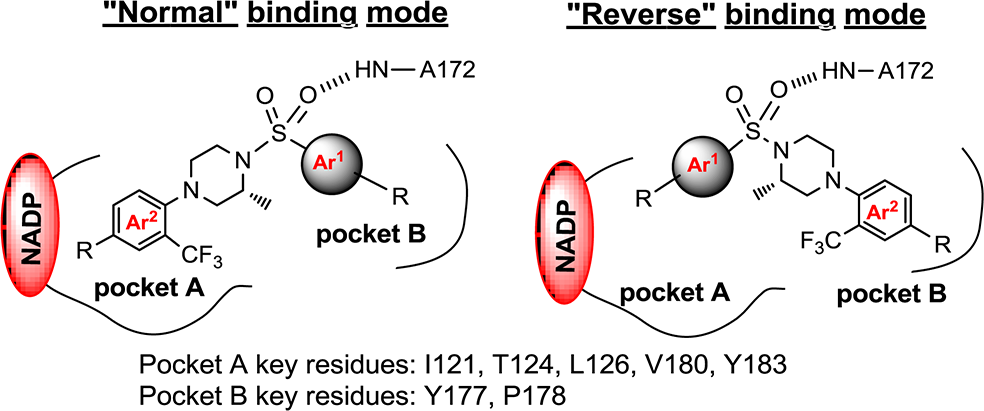
“Normal” and “reverse” binding modes of piperazine sulfonamides as 11β-HSD1 inhibitors in human 11β-HSD1 enzyme.
CAS number: 591-50-4
Iodobenzene is defined as an aromatic compound that serves as a starting material for the preparation of hypervalent iodine reagents, often alongside ring-substituted analogues such as iodobenzoic acid.
Recycling of catalyst for the reaction of iodobenzene with phenylacetylene.
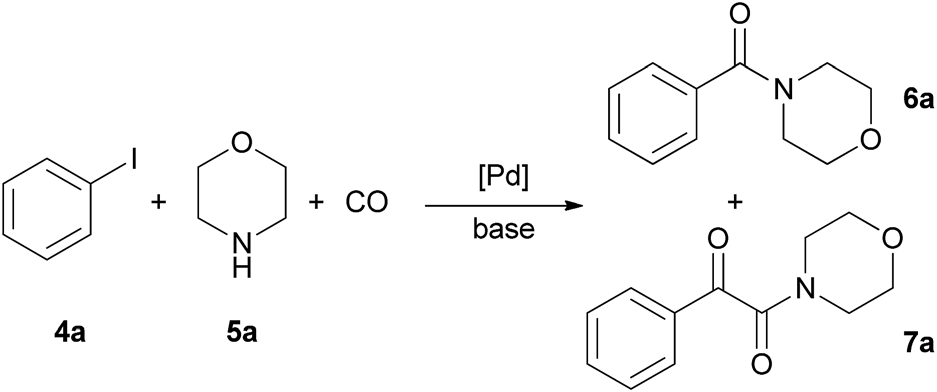
Aminocarbonylation of iodobenzene with morpholine.
CAS number: 591-59-3
Glyceraldehyde 3-phosphate is an aldotriose phosphate that is the 3-phospho derivative of glyceraldehyde. It is an important metabolic intermediate in several central metabolic pathways in all organisms. It has a role as a human metabolite, a plant metabolite and an Escherichia coli metabolite. It is an aldotriose phosphate and an aldehyde. It is functionally related to a glyceraldehyde. It is a conjugate acid of a glyceraldehyde 3-phosphate(2-).
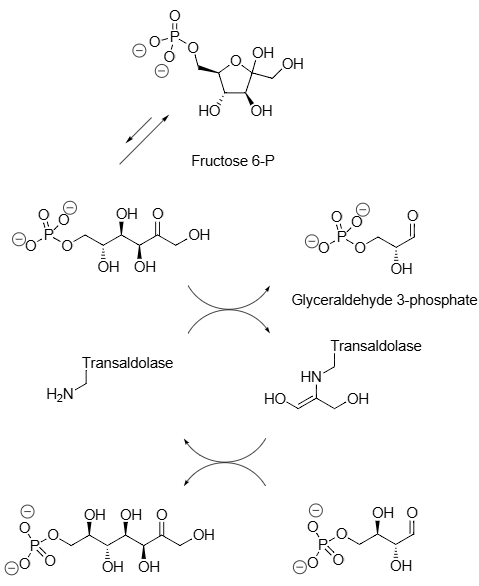
Natural reactions catalyzed by transaldolases: Erythrose 4-phosphate, Sedoheptulose 7-phosphate, Glyceraldehyde 3-phosphate.
CAS number: 591-87-7
Allyl acetate is a flammable liquid and poses a fire hazard. Allyl acetate is a colorless, water-insoluble liquid with a pungent odor.
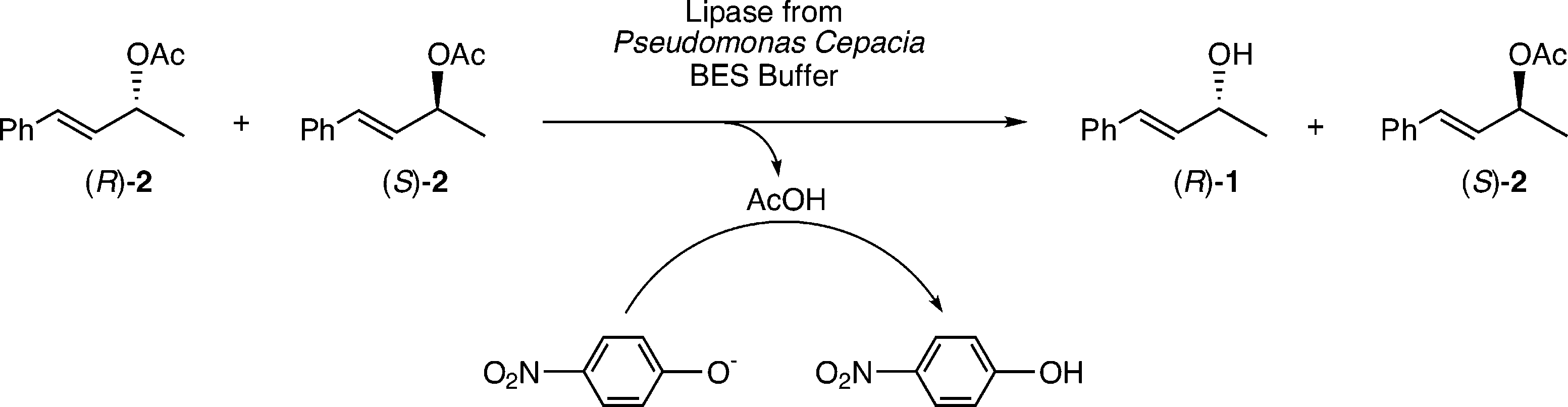
EMDee assay of allylic acetate 2 using the lipase from Pseudomonas cepacia.
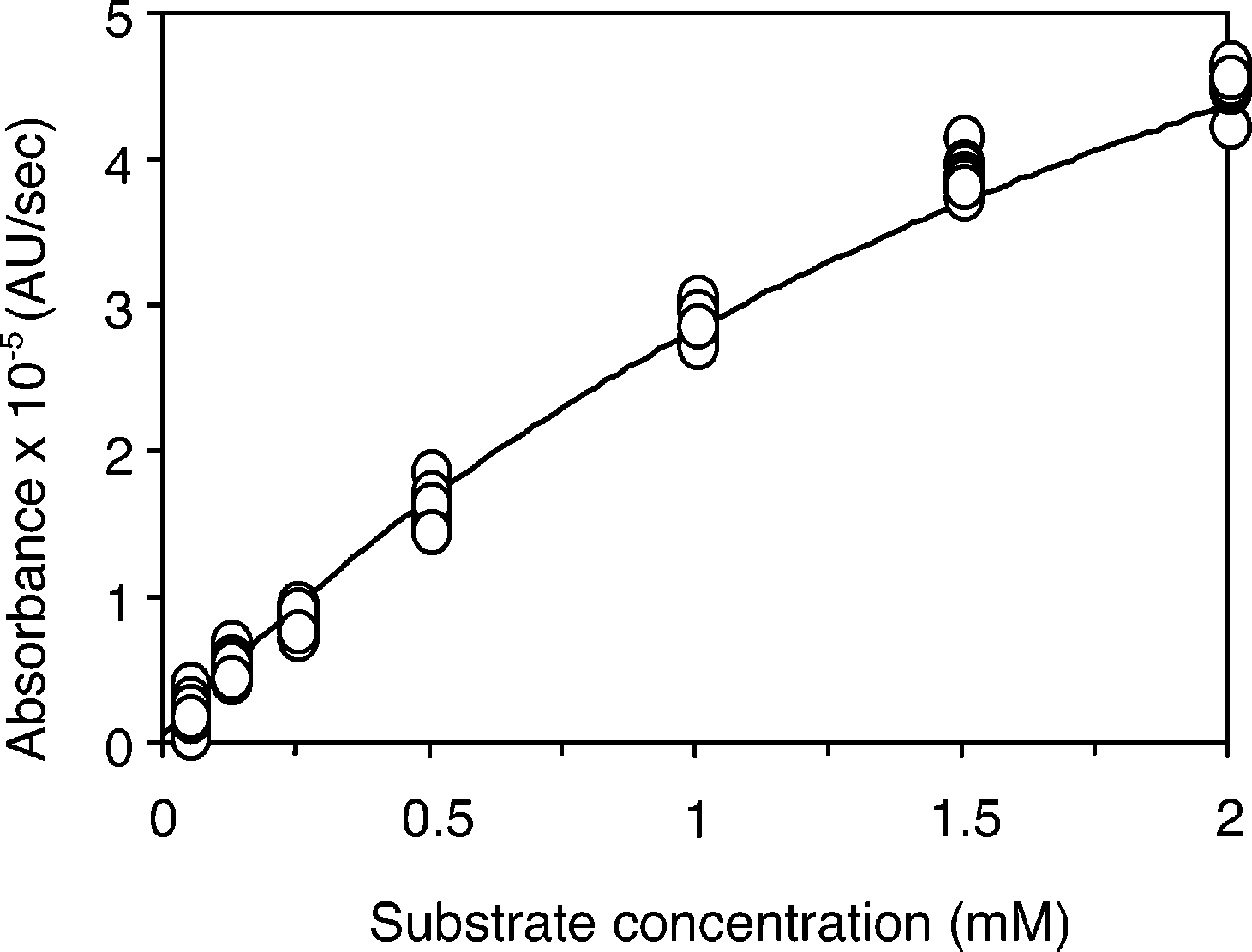
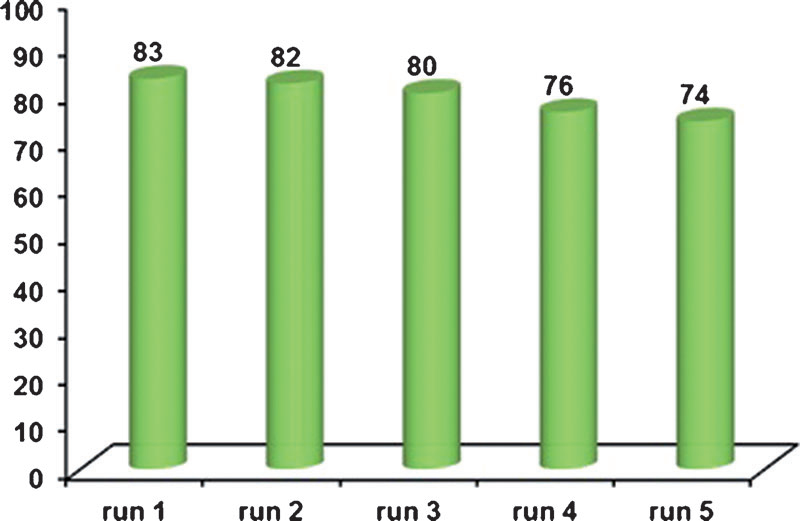
Plot of the rate of the lipase-catalyzed reaction as a function of the concentration of (R)-2.
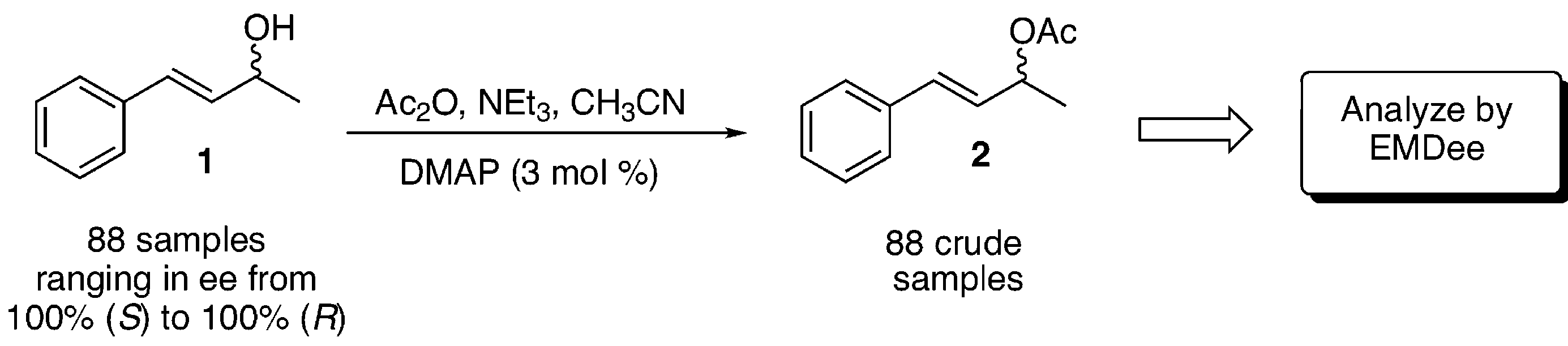
Preparation and EMDee analysis of 88 samples of crude allylic acetate 2.
CAS number: 591-93-5
1,4-Pentadiene is an organic compound characterized by the presence of two carbon-carbon double bonds, one at the 1st and another at the 4th carbon position. It's also known as penta-1,4-diene.
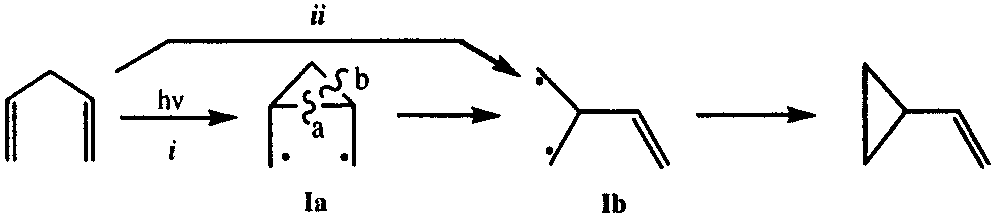
Di-π-methane Rearrangement Reaction Mechanisms for 1,4-Pentadiene: (i) Zimmerman Mechanism and (ii) Bernardi and Robb Mechanism
CAS number: 592479-02-2
1-(2,2-Dimethoxy-ethyl)-2-isocyano-benzene features a benzene ring substituted with a 2,2-dimethoxyethyl group and an isocyano group.
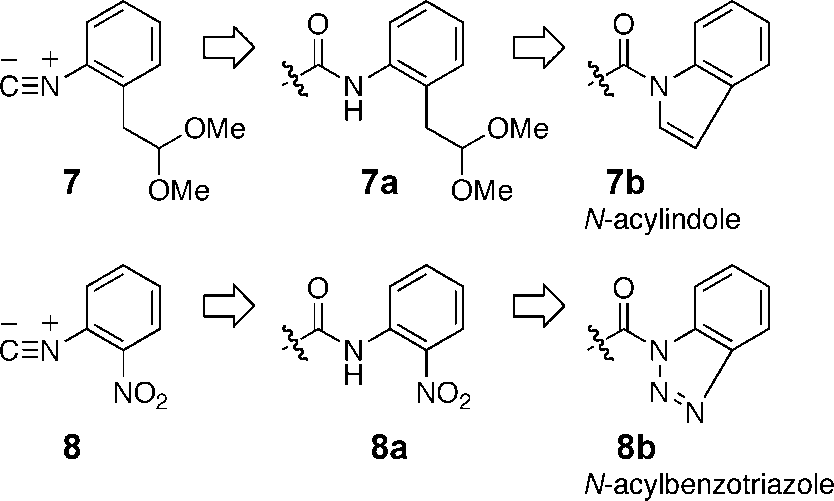
Structures of convertible isocyanides 1-isocyano-2-(2,2-dimethoxyethyl)benzene (7) and 2-nitrophenyl isocyanide (8).
CAS number: 593-51-1
Methylamine hydrochloride is obtained by heating two equivalents of formaldehyde (as formalin) with ammonium chloride at about 100°.
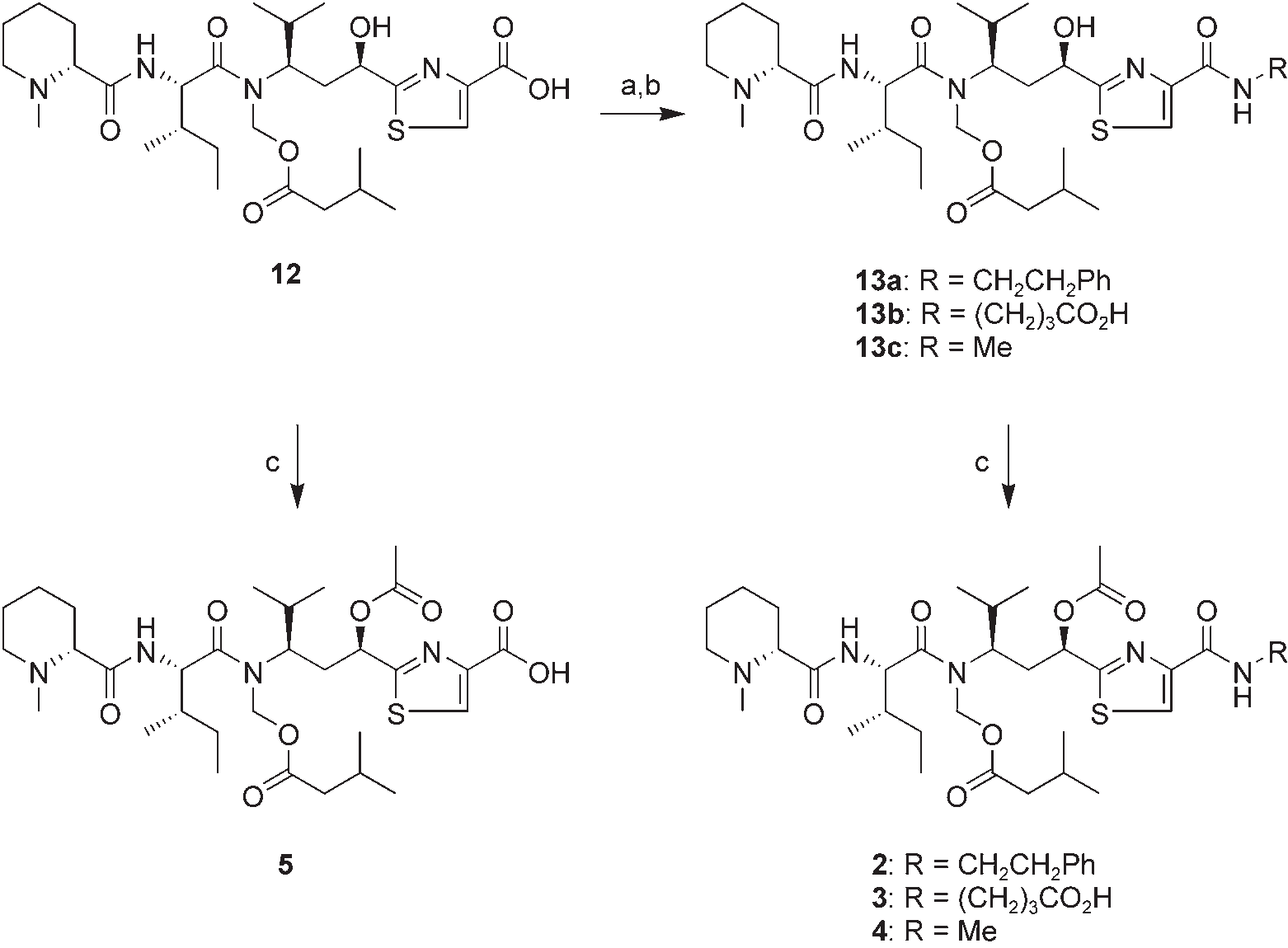
Preparation of 2–5. a) pentafluorophenol, 1,3-diisopropylcarbodiimide, CH2Cl2; b) 2-phenylethylamine, 4-aminobutyric acid, or methylamine hydrochloride, iPr2EtN, DMF, 49% (13a), 70% (13b), or 68% (13c) yield for two steps; c) Ac2O, pyridine, then (for 4 and 6) H2O/dioxane, 99% (3), 81% (4), 90% (5), or 97% (6).
CAS number: 593-60-2
Bromoethane is a bromoalkane that is ethane carrying a bromo substituent.
![Highly Z-selective olefination. [a] Vinyl bromide (1.05 equiv), tBuLi (2.1 equiv), THF, À788C; then boronic ester (1.0 equiv), THF, À788C; then PhSeCl (1.2 equiv), THF, À788C to RT; then NaOMe (5.0–20.0 equiv), THF/MeOH (1:1), 08C or RT. [b] PhSe- SePh removed by oxidation with H2O2. [c] Ate complex formed with 16 and MeLi (1.05 equiv).](http://www.wlxkc.cn/picture/305605_12.png)
Highly Z-selective olefination. [a] Vinyl bromide (1.05 equiv), tBuLi (2.1 equiv), THF, À788C; then boronic ester (1.0 equiv), THF, À788C; then PhSeCl (1.2 equiv), THF, À788C to RT; then NaOMe (5.0–20.0 equiv), THF/MeOH (1:1), 08C or RT. [b] PhSe- SePh removed by oxidation with H2O2. [c] Ate complex formed with 16 and MeLi (1.05 equiv).
CAS number: 593-66-8
Iodoethene, also known as vinyl iodide, is an organic compound features a vinyl group (CH₂=CH-) with an iodine atom attached to one of the carbon atoms.
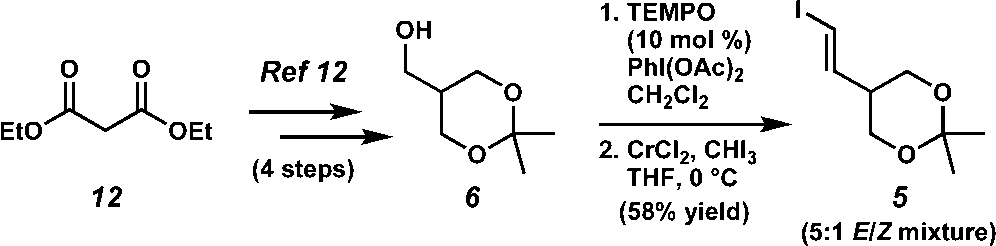
Synthesis of vinyl iodide 5
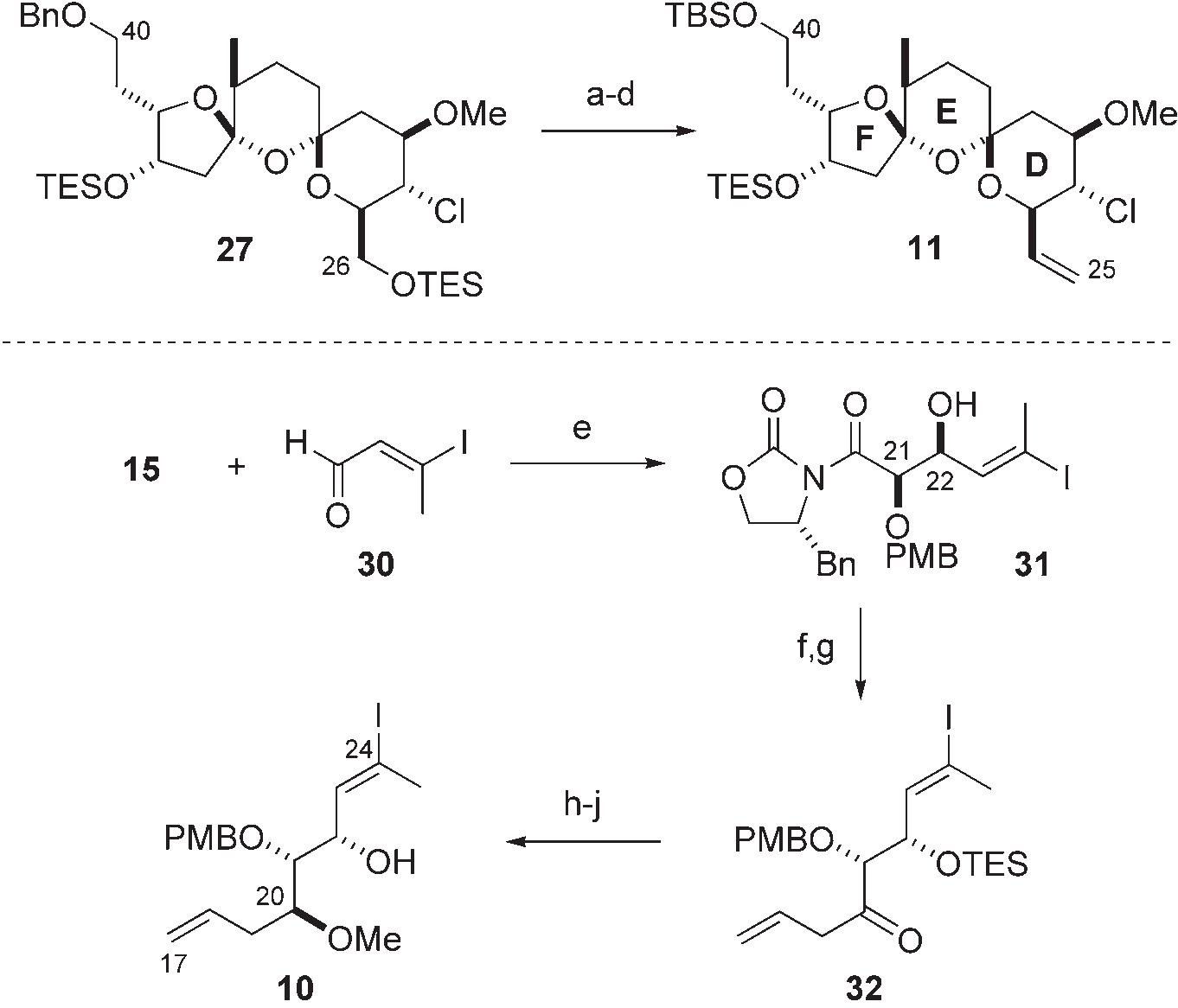
Preparation of the C25–C40 alkene 11 and the C17–C24 vinyl iodide 10.
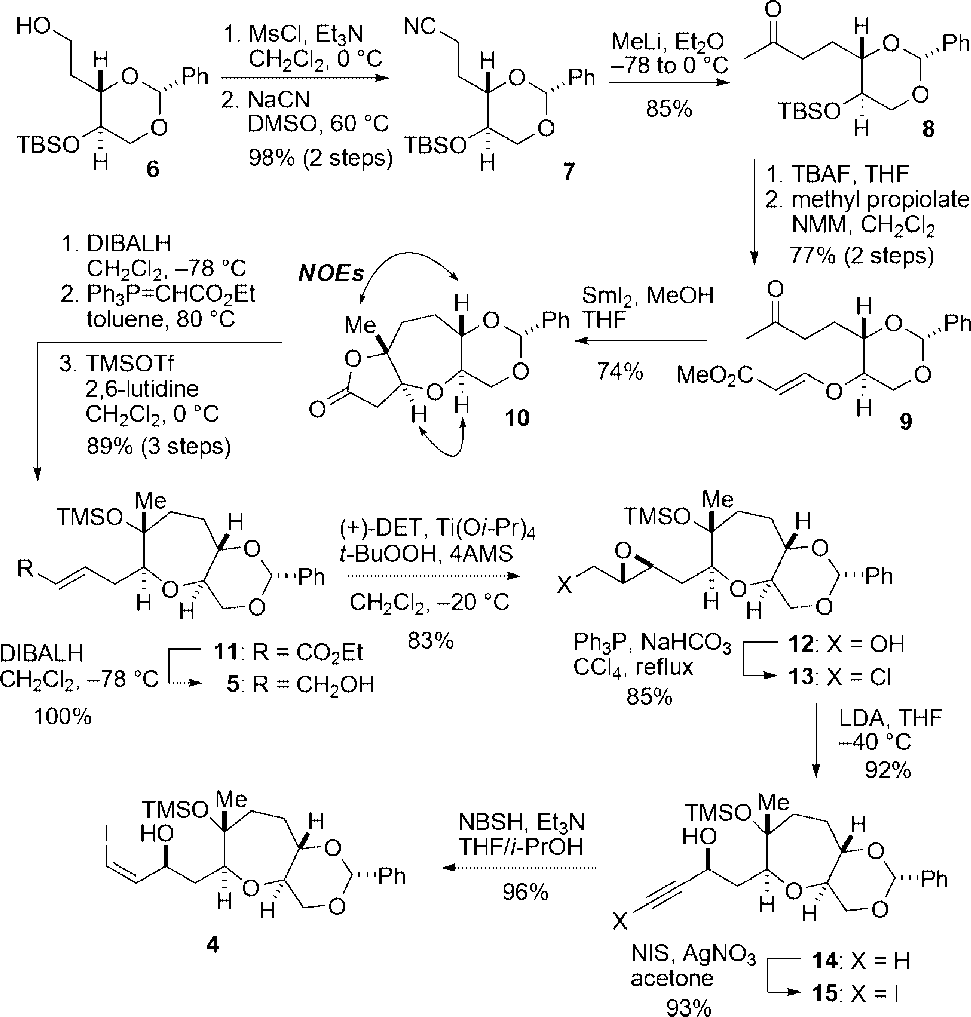
Synthesis of Vinyl Iodide 4