Porphyrin
CAS number: 101-60-0
Porphyrin is a group of organic heterocyclic compounds that are comprised of four modified pyrrole subunits connected via methine bridges and form an aromatic macrocyclic structure, which has one or more side chains attached. Many porphyrins are naturally occurring pigments. Porphyrins with metal ions bound to the porphin ring can be cofactors for biological processes, such as oxygen transport by hemes and photosynthesis by chlorophyll.
Related images
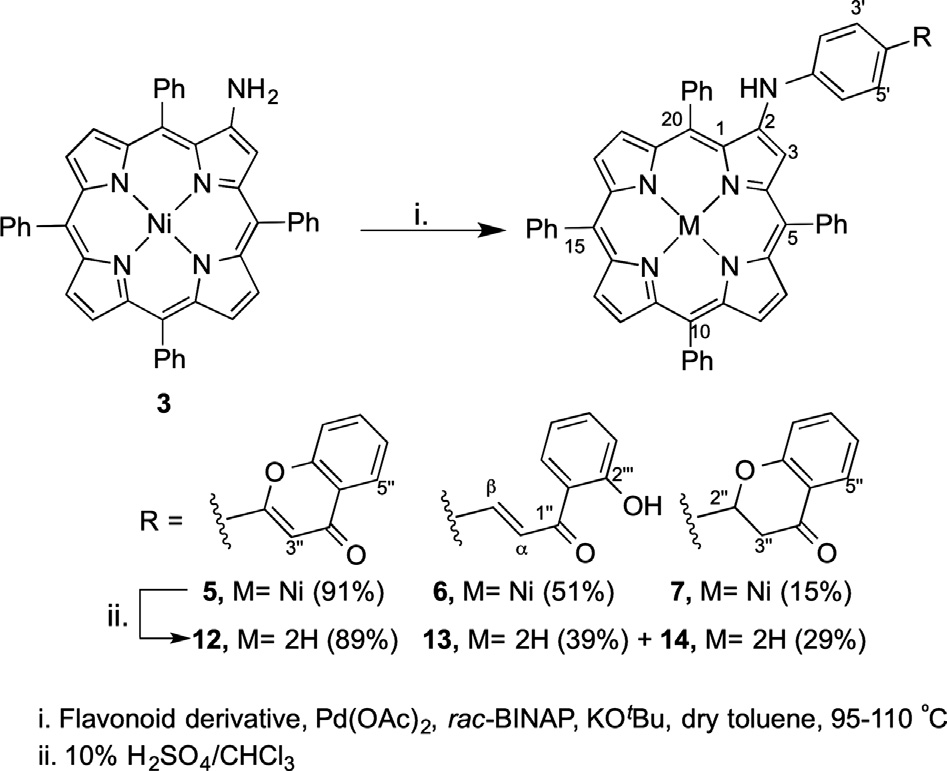
Synthetic strategy to prepare flavonoid–porphyrin conjugates at β-position.
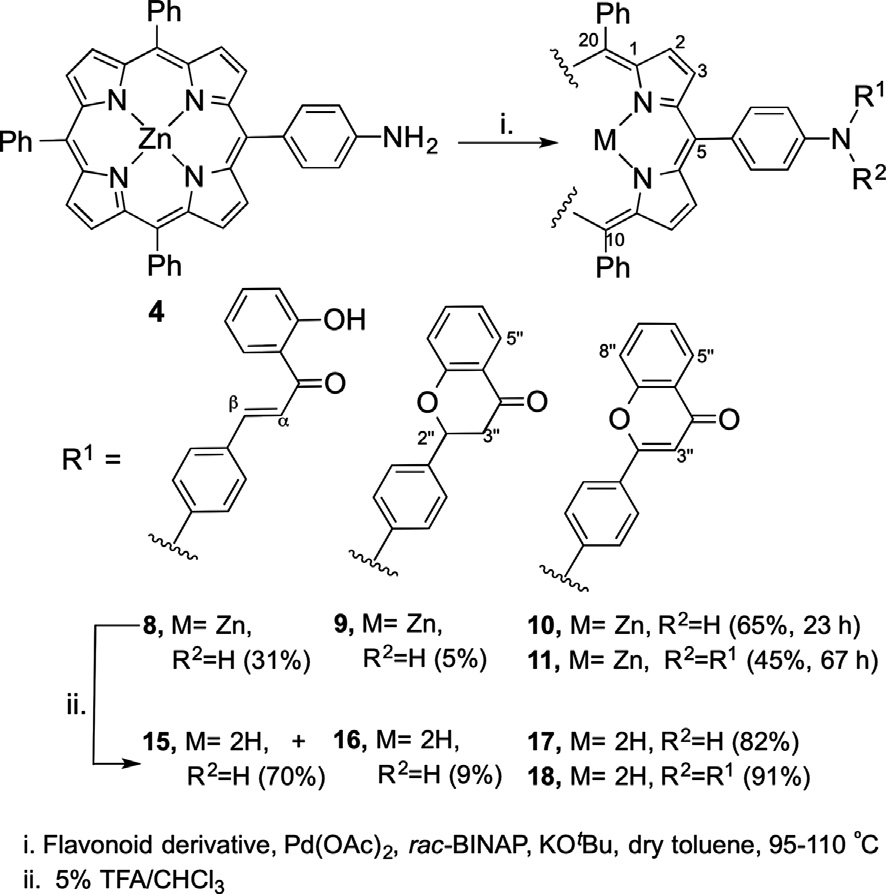
Synthetic strategy to prepare flavonoid–porphyrin conjugates at meso-position.
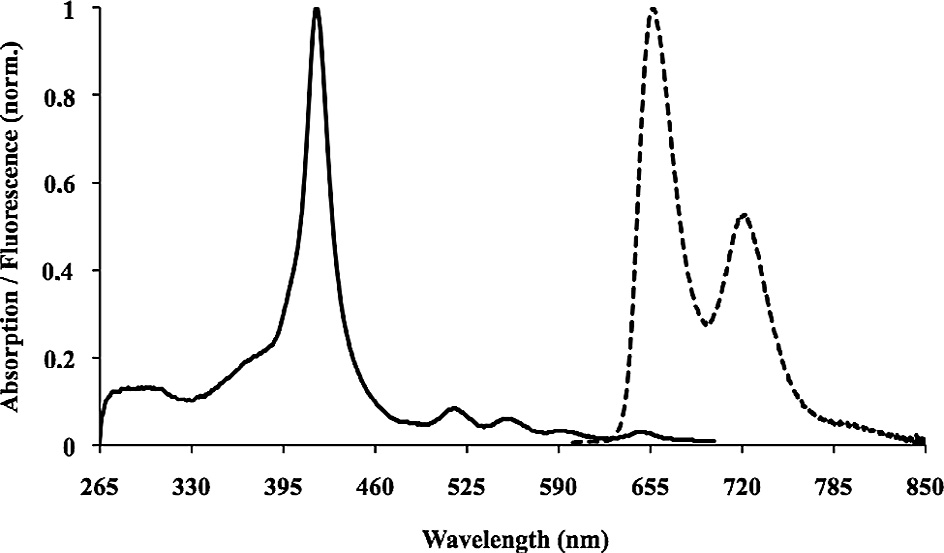
Representative normalized absorption (solid) and fluorescence spectra (dotted) (kexc = 550 nm, OD = 0.02) of flavonoid–porphyrin conjugate 17 in DMF.
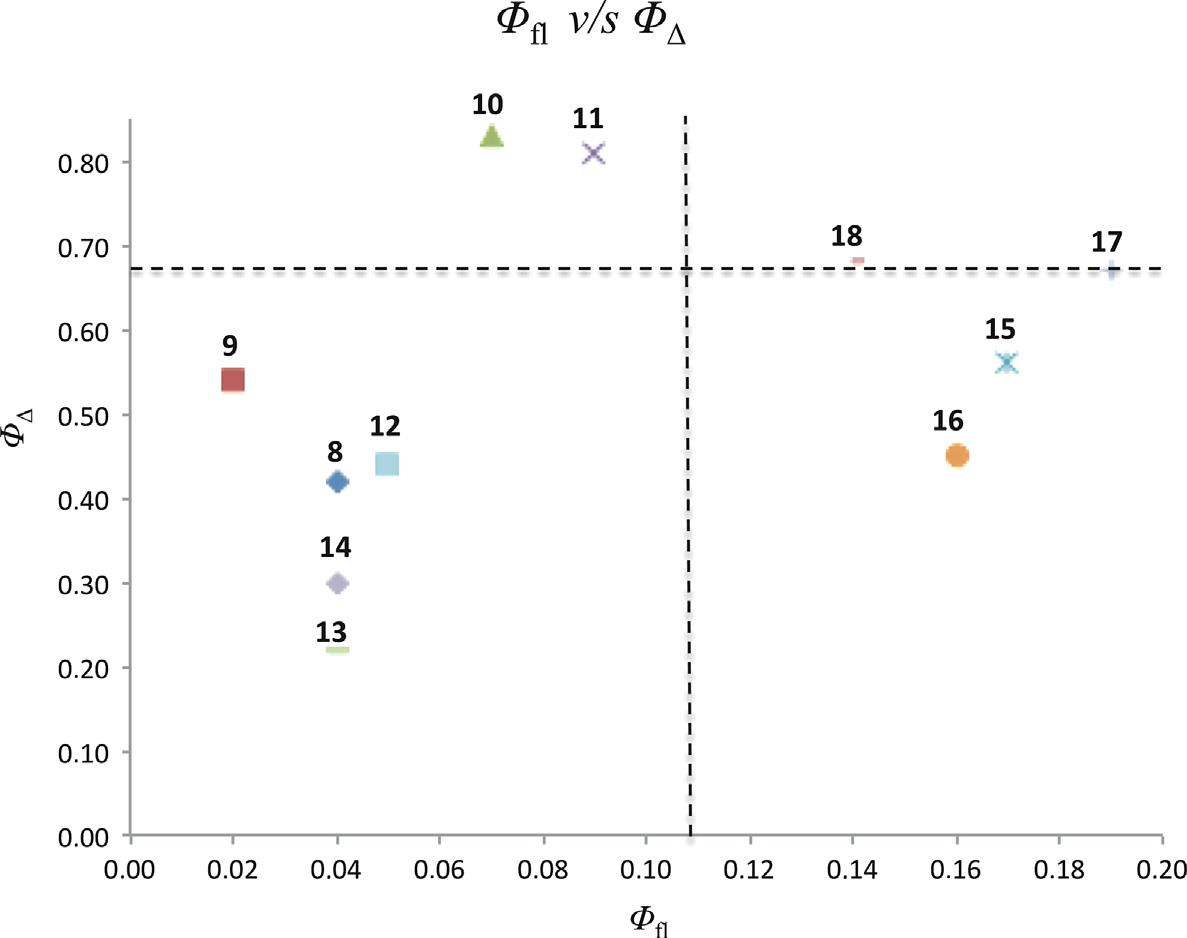
Plot of the singlet oxygen quantum yield (Ø△) of the flavonoid–porphyrin conjugates versus their fluorescence quantum yield (Øfl) in DMF. The interception of the dotted lines represents the value of the standard (TPP).
Related Questions and Answers
A: The porphyrin–azoheteroarene hybrids exhibit higher fluorescence emission intensities, more significant Stokes shifts, and smaller optical band gaps compared to their precursor porphyrins. For example, the fluorescence quantum yield of hybrid 4 (0.23) and hybrid 7 (0.18) were significantly higher than their precursor porphyrin 1 (0.093).
A: Peripheral substituents significantly influence the dipole moment and charge distribution in porphyrin-VOC interactions. For example, MnClTPP [p-PhSO2] has the highest dipole moment (8.9 debye) among the studied porphyrins, leading to strong polarization and high reactivity with VOCs. Phenylsulfonyl groups (p-PhSO2) exert strong electron-withdrawing effects, reducing the π-electron density and enhancing the oxidation of the Mn center, thereby improving colorimetric sensing performance. In contrast, phenylmethyl groups (p-CH3) have minimal influence due to their weak electron-withdrawing capability.
A: Metal centers, such as Mn2+ and Co2+, play a critical role in the responsiveness of porphyrin structures to VOCs. MnTPP exhibits higher binding energy and stronger interaction with VOCs compared to CoTPP. This is attributed to the strong reactivity, low ionization energy, and high reducibility of manganese atoms, making MnTPP more sensitive to VOCs. The metal center's electronic characteristics and spatial arrangement influence the overall binding affinity and colorimetric sensing performance.
A: The binding energy of porphyrins with VOCs is influenced by the central metal atom, axial ligands, and peripheral substituents. For example, MnTPP has a higher binding energy (−28.15 kcal/mol) compared to CoTPP (−26.86 kcal/mol), indicating stronger interaction. Porphyrins with peripheral substituents like p-PhSO2 (MnClTPP [p-PhSO2], −25.95 kcal/mol) exhibit higher binding energies than those with p-CH3 (MnClTPP [p-CH3], −25.51 kcal/mol). The presence of axial ligands such as Cl− and OAc− reduces the binding energy, likely due to spatial hindrance.
A: Porphyrins are heterocyclic precursor molecules essential for heme biosynthesis, a process occurring in virtually every mammalian cell.