Chemicals list & Research Gallery
CAS number: 56-41-7
L-Alanine is a metabolite found in or produced by Escherichia coli.
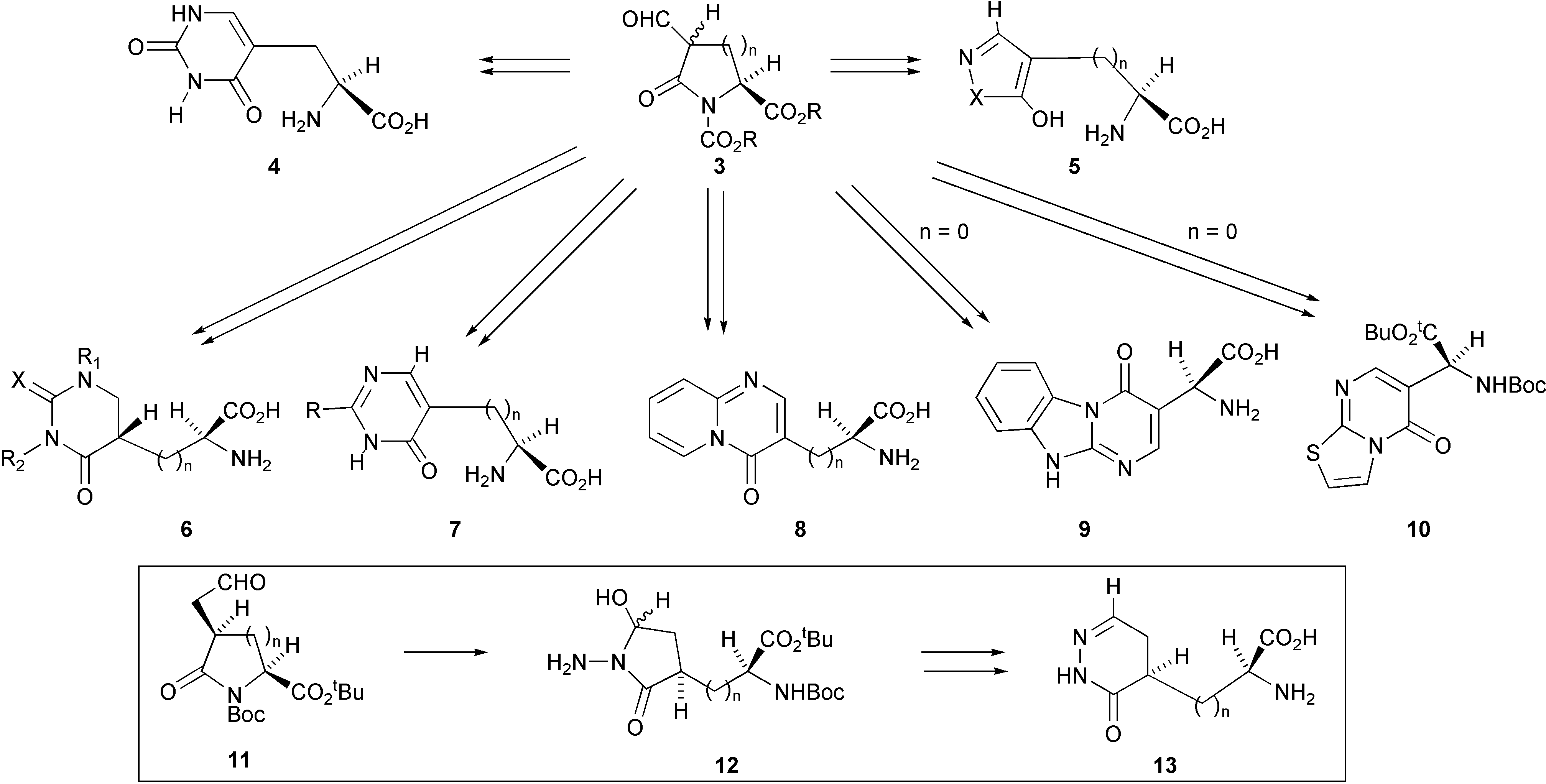
We have discovered a versatile and economical synthesis for the preparation of a large variety of homochiral compounds in which a heterocyclic ring system is fused to the β-carbon atom of L-alanine. This methodology has recently been extended to encompass the preparation of analogues of the lower homologue, ibotenic acid. The synthesis, shown in Scheme 1, involves reaction of bisnucleophiles with an aldehyde of a pyroglutamic acid derivative 3 (n = 1) or an aldehyde of a β-lactam 3 (n = 0) or with their homologues 11.
CAS number: 56-45-1
L-serine is the L-enantiomer of serine. It has a role as a human metabolite, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a serine family amino acid, a proteinogenic amino acid, a L-alpha-amino acid and a serine. It is a conjugate base of a L-serinium. It is a conjugate acid of a L-serinate. It is an enantiomer of a D-serine. It is a tautomer of a L-serine zwitterion.
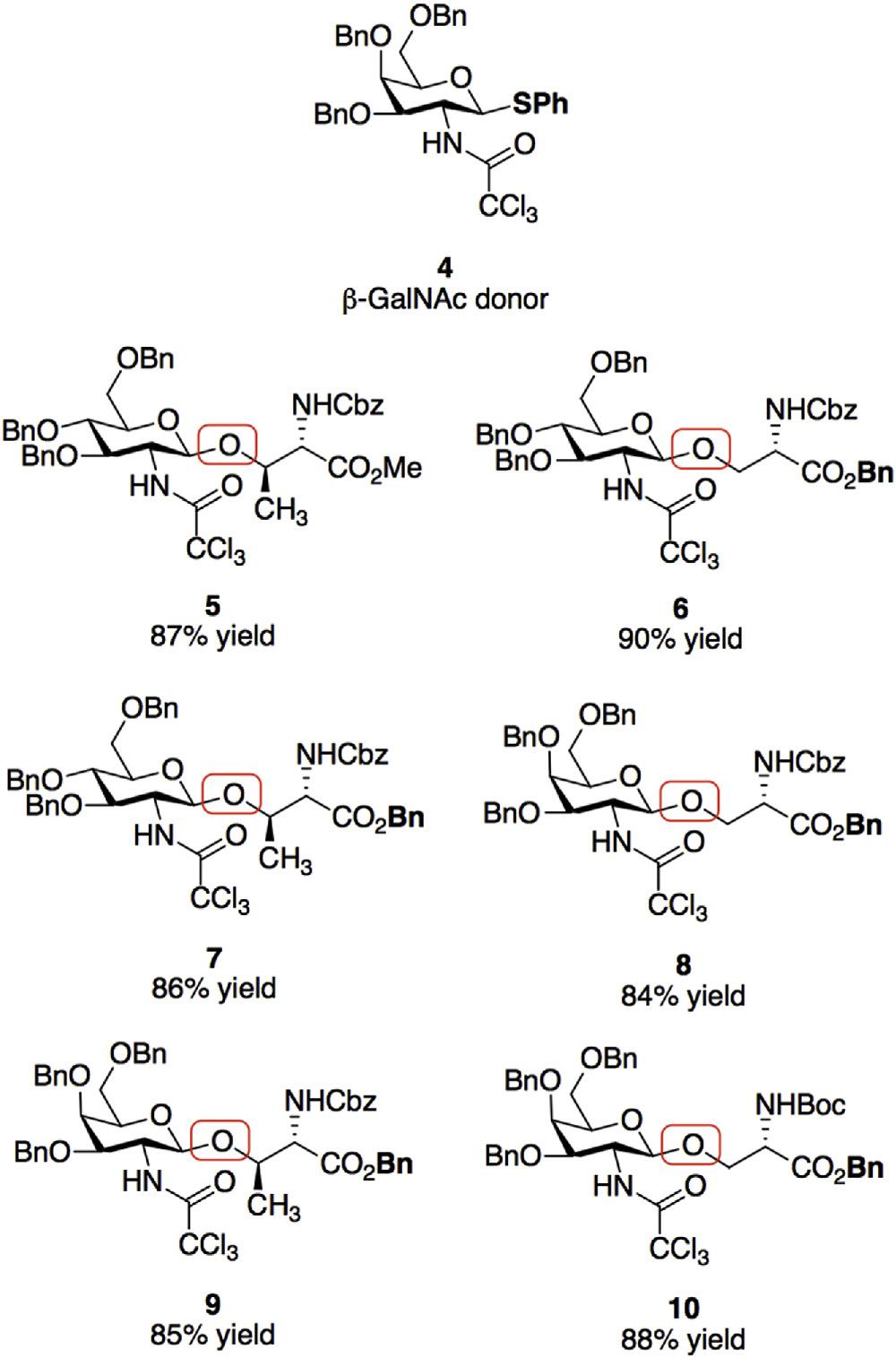
O-Glycosylation of serine hydroxyls.
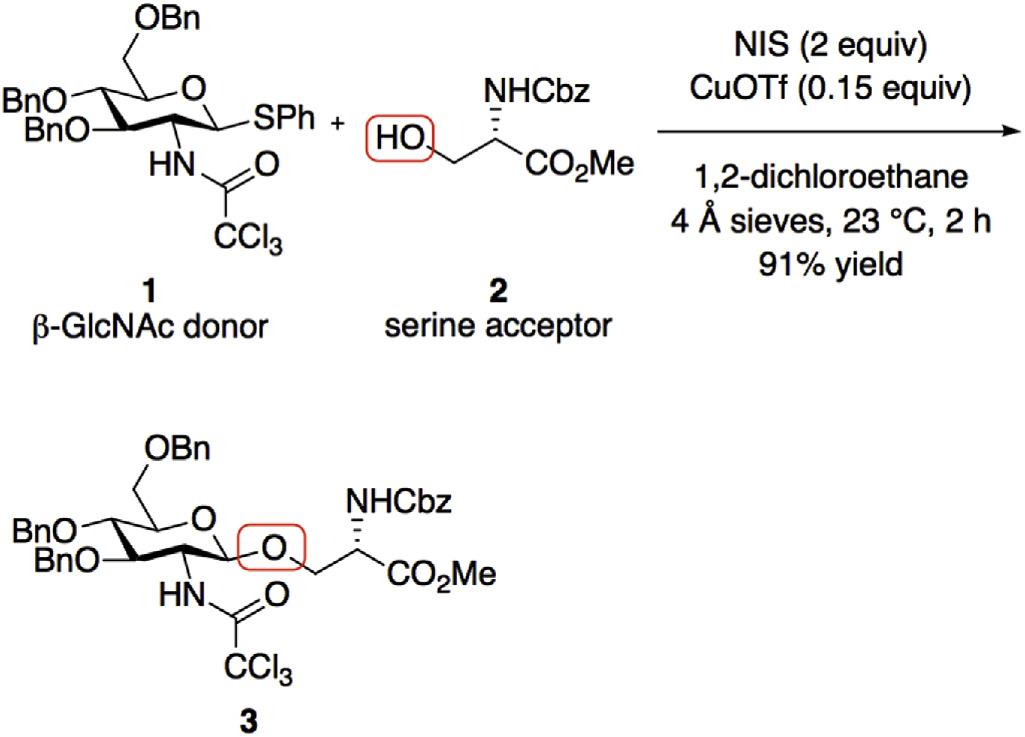
Prototypical serine O-glycosylation.
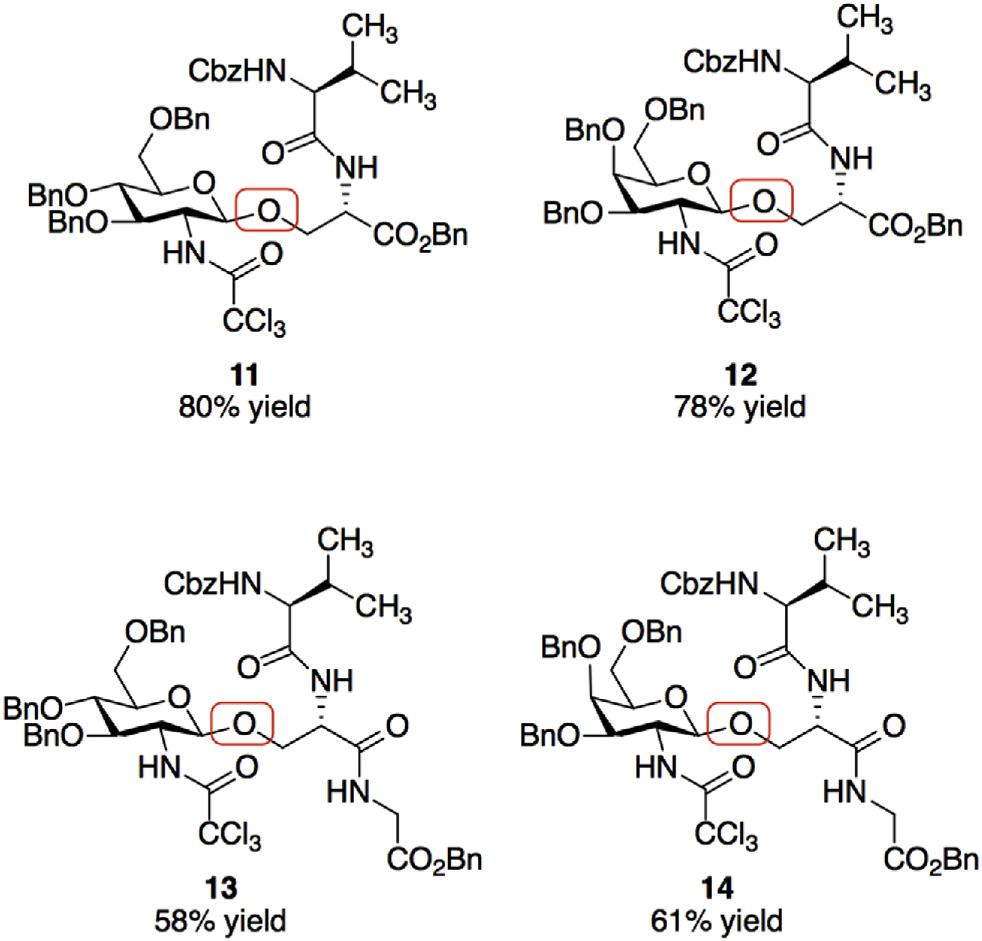
Dipeptide and tripeptide serine O-glycosylations.
CAS number: 56-54-2
Quinidine is a D-isomer of [quinine] present in the bark of the Cinchona tree and similar plant species. This alkaloid was first described in 1848 and has a long history as an antiarrhythmic medication. Quinidine is considered the first antiarrhythmic drug (class Ia) and is moderately efficacious in the acute conversion of atrial fibrillation to normal sinus rhythm. It prolongs cellular action potential by blocking sodium and potassium currents.
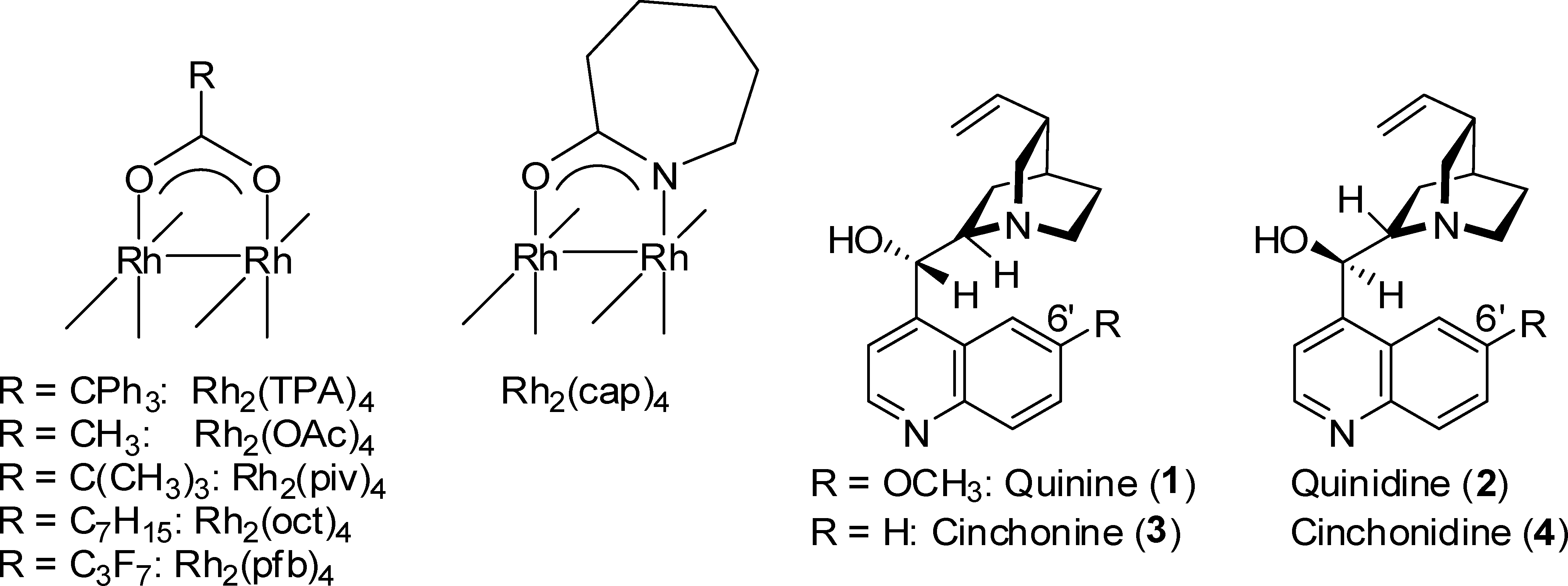
Structures of Rh(II) Complexes and Cinchona Alkaloids
CAS number: 56-81-5
Glycerol is a triol with a structure of propane substituted at positions 1, 2 and 3 by hydroxy groups. It has a role as an osmolyte, a solvent, a detergent, a human metabolite, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a geroprotector. It is an alditol and a triol.
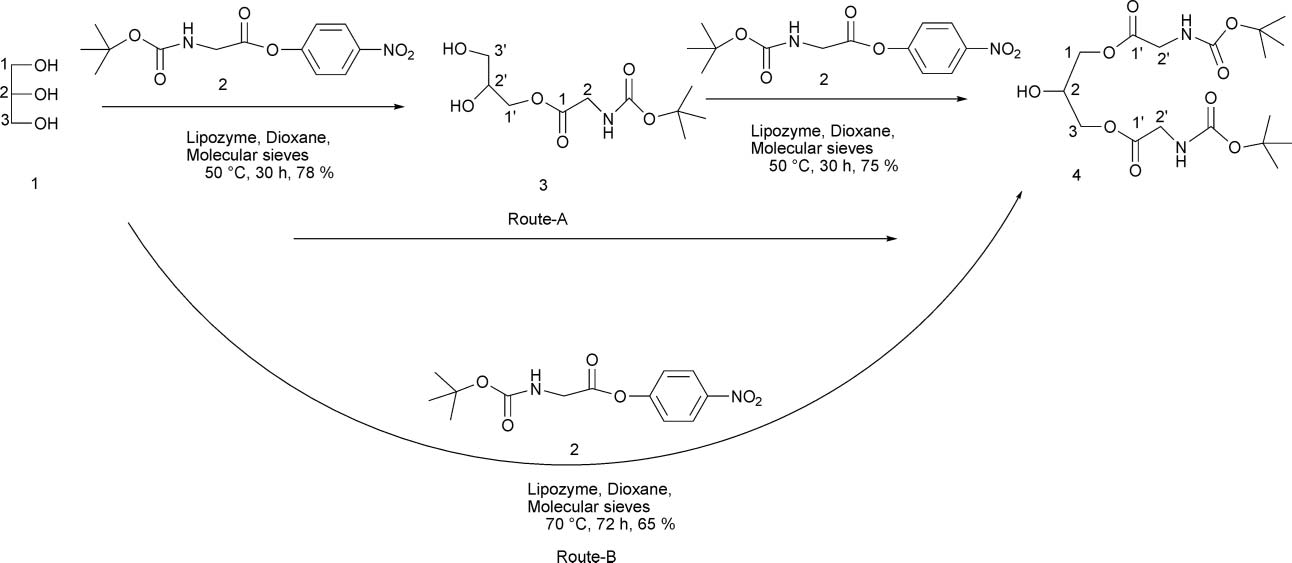
Lipozyme-catalyzed transesterification of glycerol.
CAS number: 56-86-0
L-glutamic acid is an optically active form of glutamic acid having L-configuration. It has a role as a nutraceutical, a micronutrient, an Escherichia coli metabolite, a mouse metabolite, a ferroptosis inducer and a neurotransmitter. It is a glutamine family amino acid, a proteinogenic amino acid, a glutamic acid and a L-alpha-amino acid. It is a conjugate acid of a L-glutamate(1-). It is an enantiomer of a D-glutamic acid.
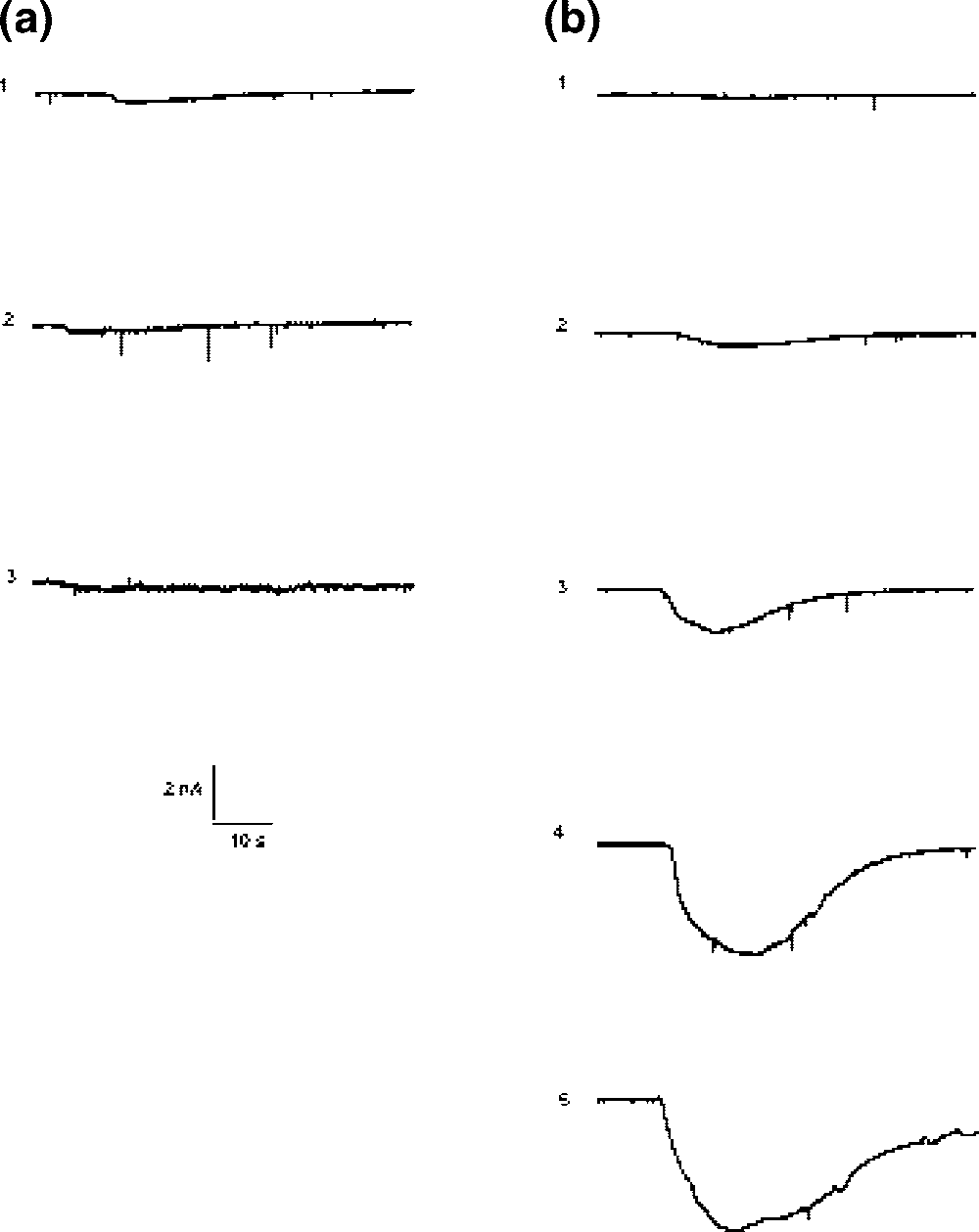
Transmembrane currents induced in Purkinje neurons by different doses of glutamate and kainic acid (KA).
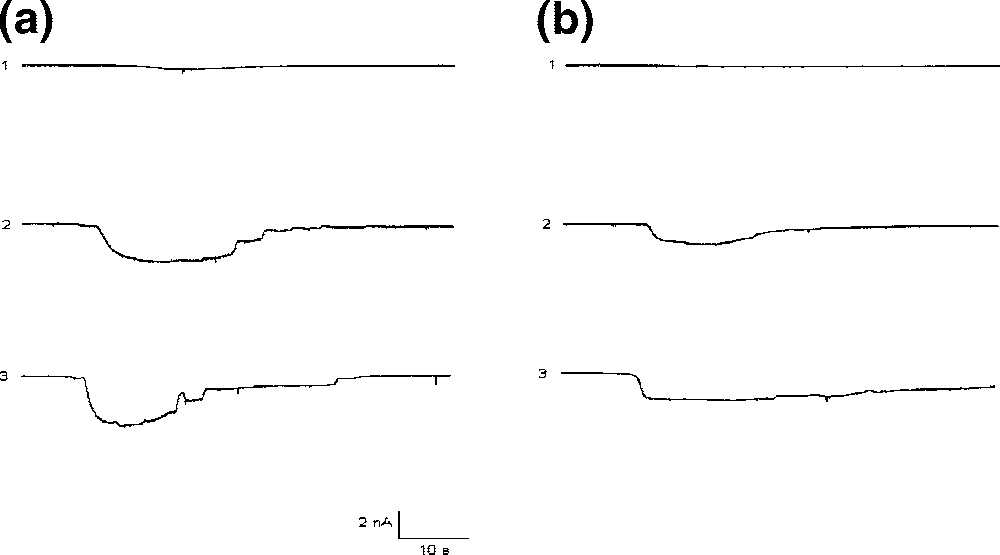
Comparative potentiation of kainic acid (KA) and glutamate responses in Purkinje neurons by CT and 3.
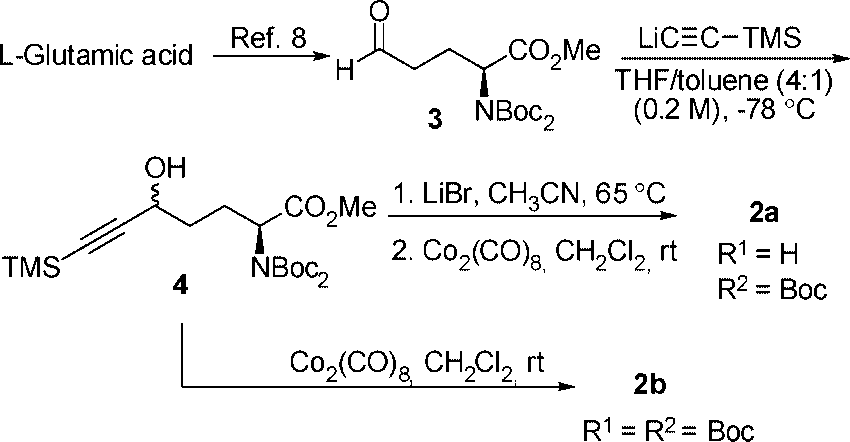
Synthesis of Co2(CO)6-δ-propargylic Alcohols Derived from L-Glutamic Acid
CAS number: 56-87-1
L-lysine is an L-alpha-amino acid; the L-isomer of lysine. It has a role as a micronutrient, a nutraceutical, an anticonvulsant, an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite, a plant metabolite, a human metabolite, an algal metabolite and a mouse metabolite.
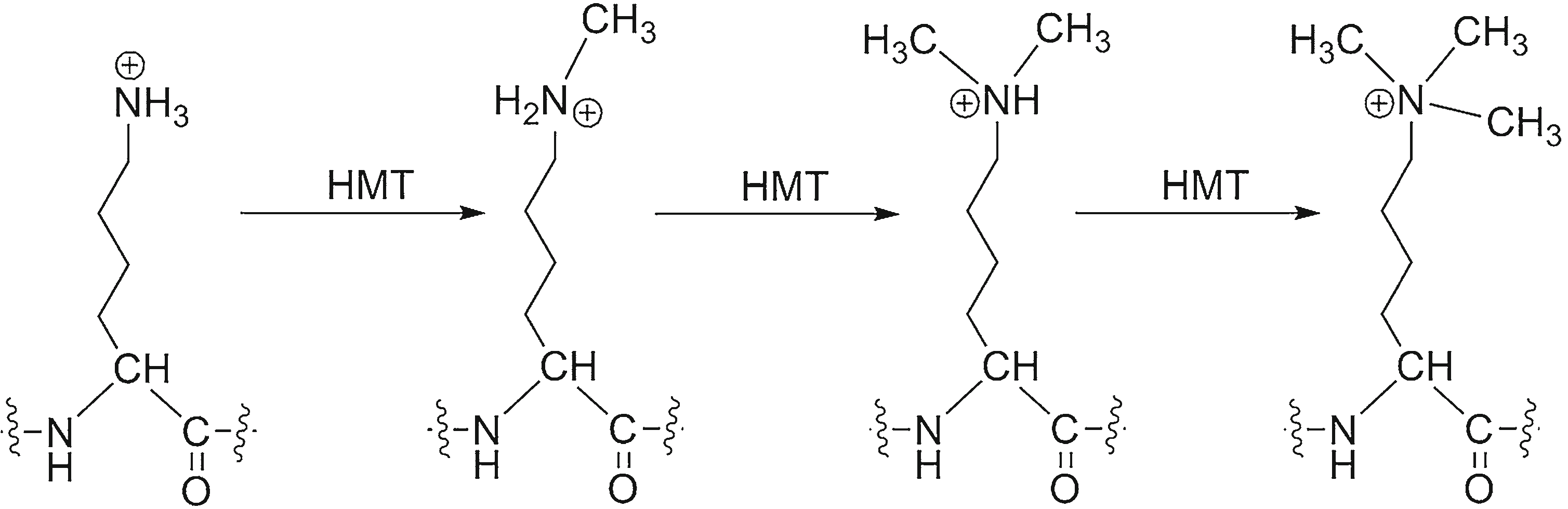
Nε-Methylation of lysine by histone methyltransferases (HMT)
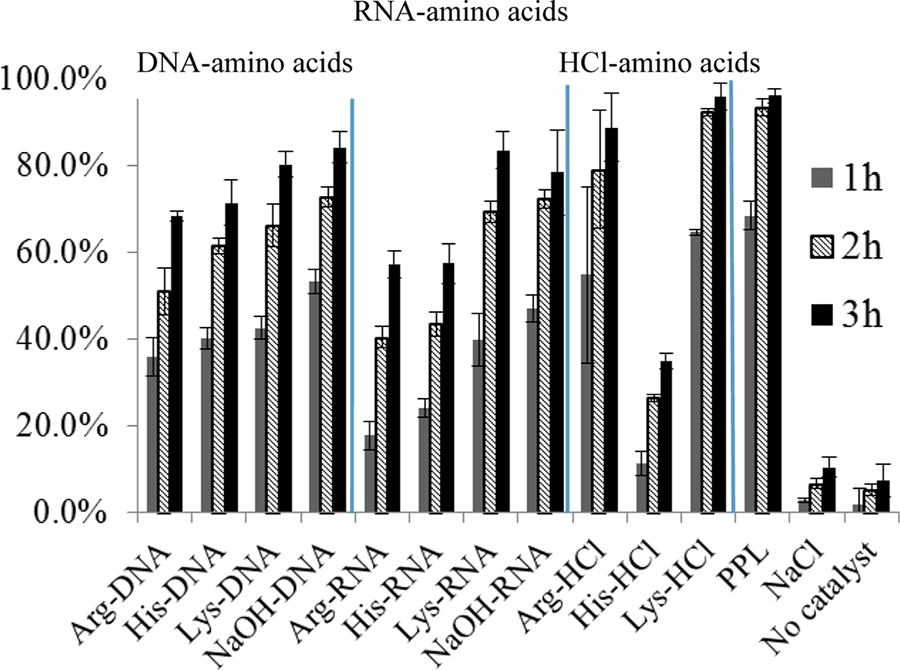
When the nucleotides were neutralized to pH 7.0 with NaOH or basic amino acids (L-lysine, L-arginine, or L-histidine), the reaction rate was significantly accelerated and the yield was comparable to that of porcine pancreatic lipase (PPL, one of the best enzymes for the Knoevenagel condensation reaction) as well as pH-neutral amino acid salts Arg•HCl and Lys•HCl.
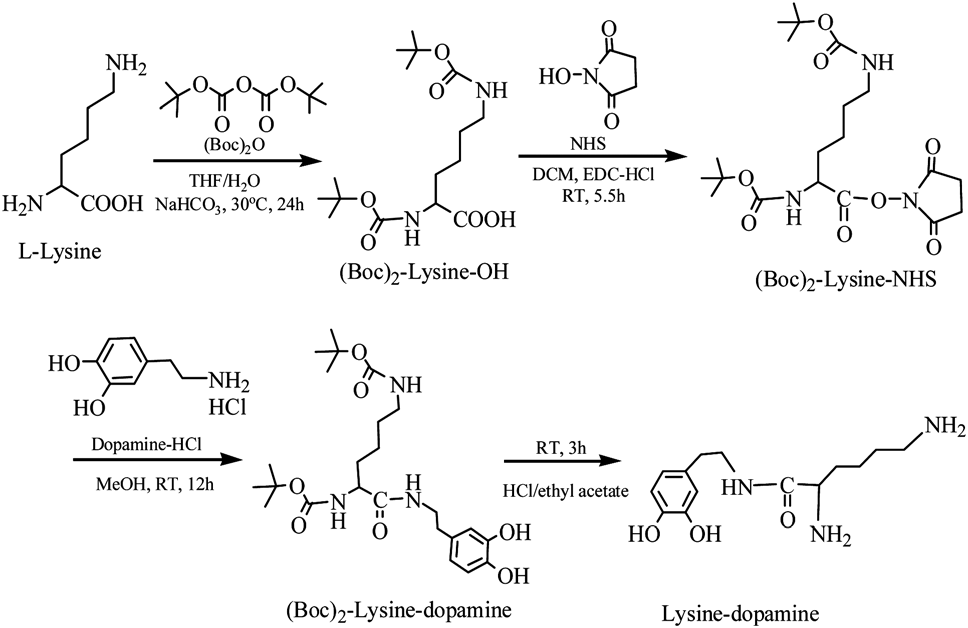
Synthesis of lysine-dopamine.
CAS number: 56038-89-2
N-(1-ethylpropyl)-3,4-dimethylaniline is an organic compound, specifically an aromatic amine, characterized by its molecular structure which includes a benzene ring with methyl groups at the 3 and 4 positions, and an N-(1-ethylpropyl) substituent.
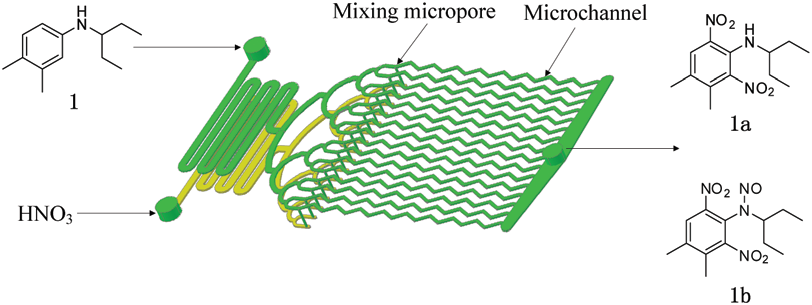
One-step dinitration of N-(1-ethylpropyl)-3,4-xylidine (1) in a continuous-flow microreactor system.
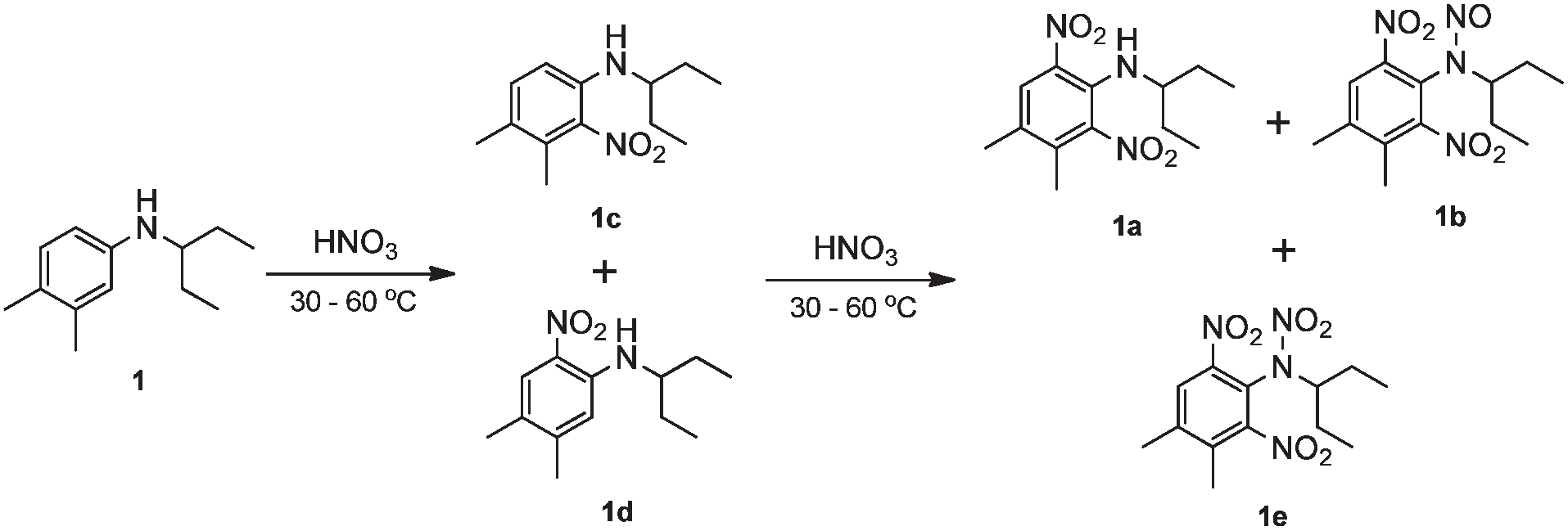
Conventional two-step dinitration of N-(1-ethylpropyl)-3,4-xylidine (1) in the batch system.
CAS number: 5604-55-7
3-Pentenal is an aldehyde with the chemical formula C5H8O, featuring a five-carbon chain with a double bond between the third and fourth carbons, and an aldehyde group at the first carbon. It exists as different stereoisomers, including the (Z)-isomer (or cis isomer) and the (E)-isomer (or trans isomer), which refer to the arrangement of the groups around the double bond.
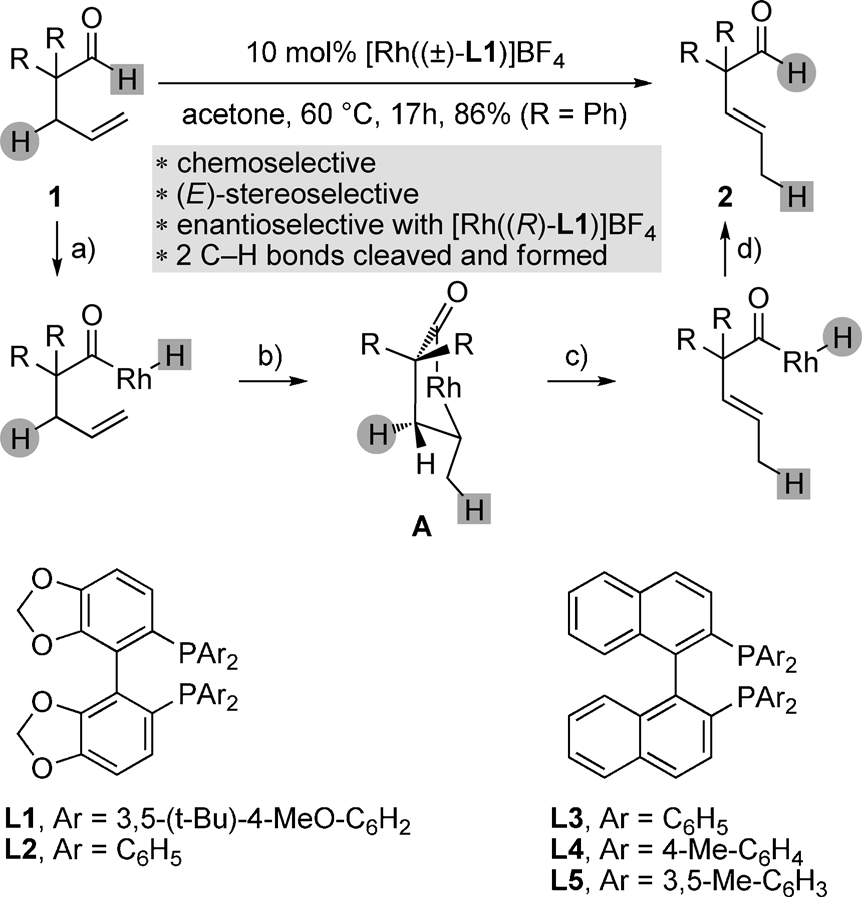
Rhodium-catalyzed isomerization of 4-pentenals into 3-pentenals by endocyclic b-H elimination.
CAS number: 5615-80-5
Aspartimide is a byproduct formed during solid-phase peptide synthesis (SPPS) of aspartic acid-containing peptides.
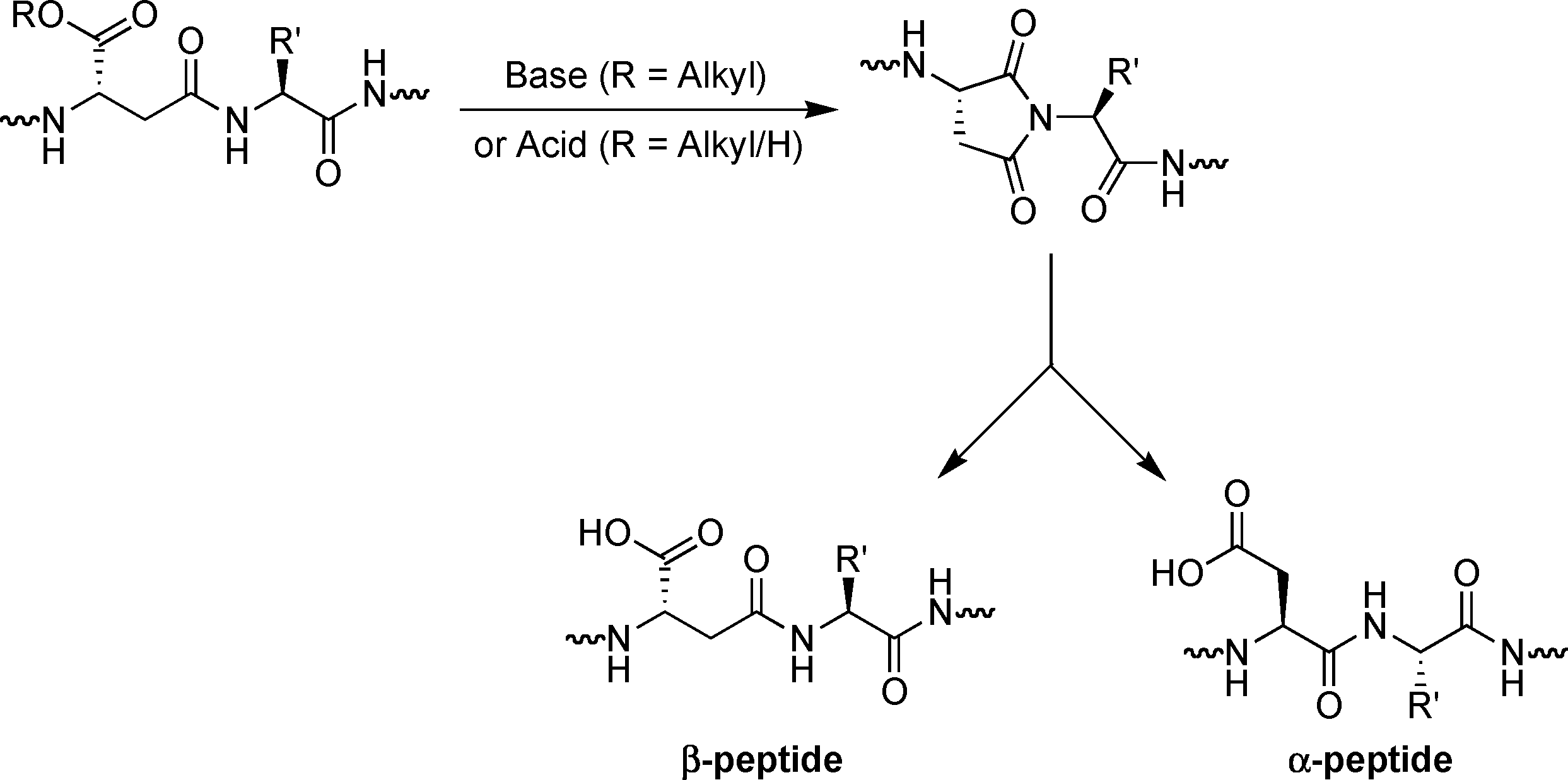
Formation of aspartimides from β-peptide derivatives.
CAS number: 56180-94-0
Acarbose is a complex oligosaccharide that acts as an inhibitor of several enzymes responsible for the breakdown of complex carbohydrates in the intestines. It inhibits both pancreatic alpha-amylase and membrane-bound alpha-glucosidases - including intestinal glucoamylase, sucrase, maltase, and isomaltase - which are responsible for the metabolism of complex starches and oligo-, tri-, and disaccharides into absorbable simple sugars. By inhibiting the activity of these enzymes, acarbose limits the absorption of dietary carbohydrates and the subsequent postprandial increase in blood glucose and insulin levels.
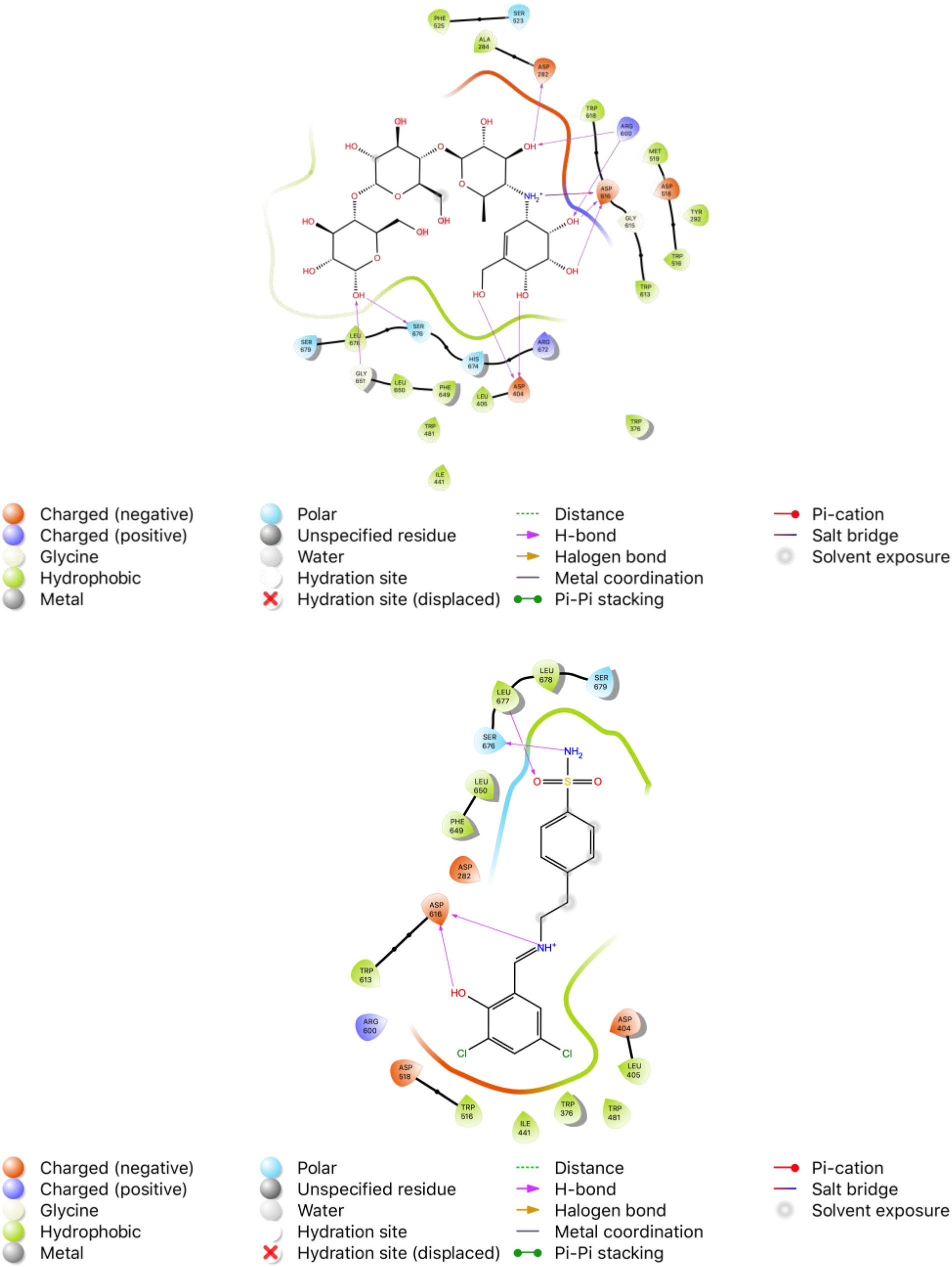
2D binding modes of native ligand α-acarbose (ACR) and compound 10 in the active site of α-GLY (PDB ID: 5NN8), respectively.