p-Dimethylaminobenzaldehyde
CAS number: 100-10-7
4-(dimethylamino)benzaldehyde is a member of the class of benzaldehydes that is benzaldehyde carrying a dimethylamino substituent at position 4. Used as an indicator for detection of indoles and hydrazine. It has a role as a chromogenic compound. It is a member of benzaldehydes, a substituted aniline and a tertiary amino compound.
Related images
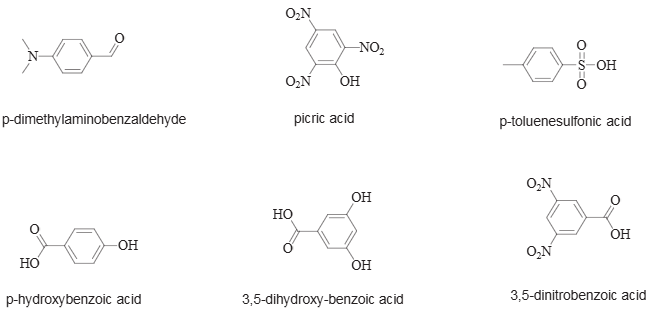
The building blocks discussed in this paper: p-dimethylaminobenzaldehyde, picric acid, p-toluenesulfonic acid, p-hydroxybenzoic acid, 3,5-dihydroxy-benzoic acid, 3,5-dinitrobenzoic acid.
Related Questions and Answers
A: The p-dimethylaminobenzaldehyde-reactive substances in the collagen of streptozotocin-diabetic rats contribute to increased cross-linking, which is associated with reduced tendon mechanical strength and increased AGE-related fluorescence. These changes are indicative of advanced glycation and may serve as a tissue measure of prolonged hyperglycemia.
A: Elevated temperatures were required for optimal coupling reactions. For ceftriaxone and cefotaxime, the optimal temperature was 50°C, while for ceftazidime, cefixime, and cefuroxime, it was 60°C. Higher temperatures increased the reaction rate and absorbance values, but temperatures beyond the optimal range led to thermal decomposition of the azo adducts, resulting in decreased absorbance.
A: Methanol was found to be the best diluting solvent for the reaction with DMAB. It provided the highest absorbance values compared to other solvents like water, ethanol, propan-1-ol, and propan-2-ol. Methanol likely stabilizes the azo adducts by creating a less polar environment, preventing hydrolytic decomposition and ensuring accurate spectrophotometric detection.
A: DMAB serves as a coupling agent that reacts with diazotized cephalosporins to form azo dyes. These azo dyes absorb light in the visible region (400–430 nm), enabling spectrophotometric analysis. The use of DMAB allows for a simple, cost-effective, and accurate method for determining cephalosporins in bulk samples and pharmaceutical dosage forms.