Benzyl alcohol
CAS number: 100-51-6
Benzyl alcohol is a colorless liquid with a mild, pleasant, aromatic odor.
Related images
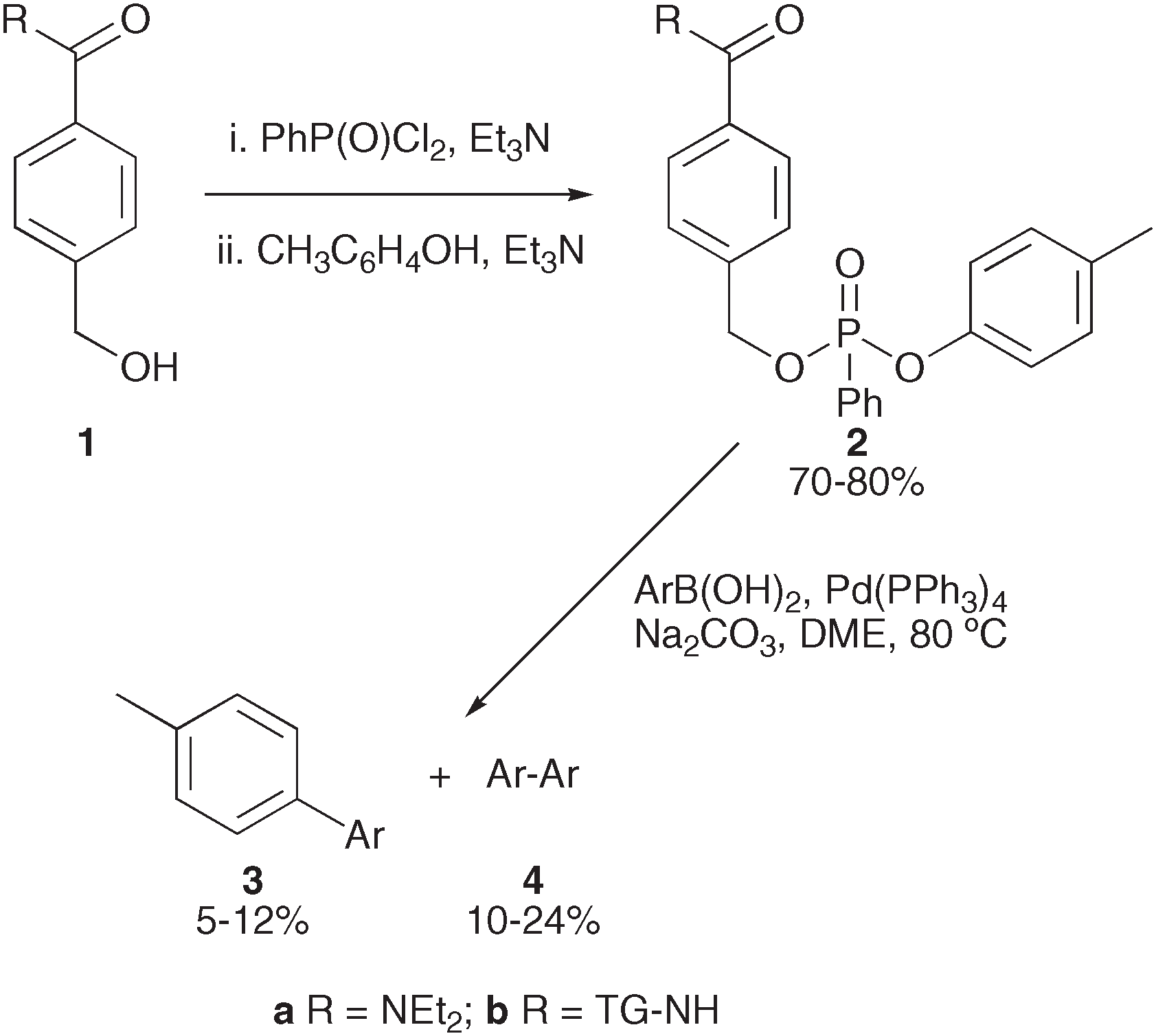
Benzyl alcohol 1a was reacted with 1.25 equivalents of phenylphosphonyl dichloride (PPDC; PhP(O)Cl2) in the presence of triethylamine (Et3N) to afford a mixture of mono- and bis-phosphates. The monosubstituted product was reacted directly with p-cresol without purification to afford the desired phenylphosphonate 2a.
Related Questions and Answers
A: The proposed photocatalytic mechanism involves the excitation of GCN in the CNTs/GCN hybrid to generate electron–hole pairs. The photogenerated electrons migrate to the CNTs and are rapidly transported away, suppressing charge recombination. The electrons are scavenged by dissolved oxygen to form superoxide radicals (·O2−), which oxidize benzyl alcohol adsorbed on the photocatalyst surface. The photogenerated holes (h+) react with benzyl alcohol to form benzaldehyde through a deprotonation process. The high selectivity for benzaldehyde is due to the thermodynamically controlled oxidation pathway linked to the energy band structure of the photocatalyst.
A: The CNTs/GCN hybrid improves the photocatalytic oxidation of benzyl alcohol in a Pickering emulsion system by serving dual roles as both a solid emulsifier and a photocatalyst. The hydrophobic CNTs enhance the affinity of benzyl alcohol to the hybrid surface, while the improved charge separation efficiency due to CNTs' conductivity leads to higher photocatalytic activity. This results in a three-fold increase in benzyl alcohol conversion efficiency compared to a traditional oil–water non-emulsion system.
A: Benzyl alcohol is used in this study as a substrate for photocatalytic hydrogen production and benzaldehyde synthesis. The oxidation of benzyl alcohol requires less energy, enabling higher hydrogen yields even at lower light intensities. Additionally, benzaldehyde, a by-product of the photocatalytic reaction, is a valuable industrial raw material used in pharmaceuticals, pesticides, and fine chemicals.
A: The Au/CuO NWs catalyst enhances benzyl alcohol electrooxidation by inducing stronger local electric fields due to its nanowire morphology. This promotes the interfacial enrichment of benzyl alkoxide and the formation of OH* species, thereby improving the overall performance. The catalyst achieved an impressive current density of 734 mA cm−2 at 1.5 V vs. RHE, with a benzyl alcohol oxidation rate of 4.74 mmol cm−2 h−1 and a 91% faradaic efficiency for benzoic acid production.