Lisinopril
CAS number: 76547-98-3
Lisinopril is an angiotensin converting enzyme inhibitor (ACEI) used to treat hypertension, heart failure, and myocardial infarction. Lisinopril and [captopril] are the only ACEIs that are not prodrugs. It functions by inhibition of angiotensin converting enzyme as well as the renin angiotensin aldosterone system. ACEIs are commonly used as a first line therapy in the treatment of hypertension, along with thiazide diuretics or beta blockers. Lisinopril was granted FDA approval on 29 December 1987.
Related images
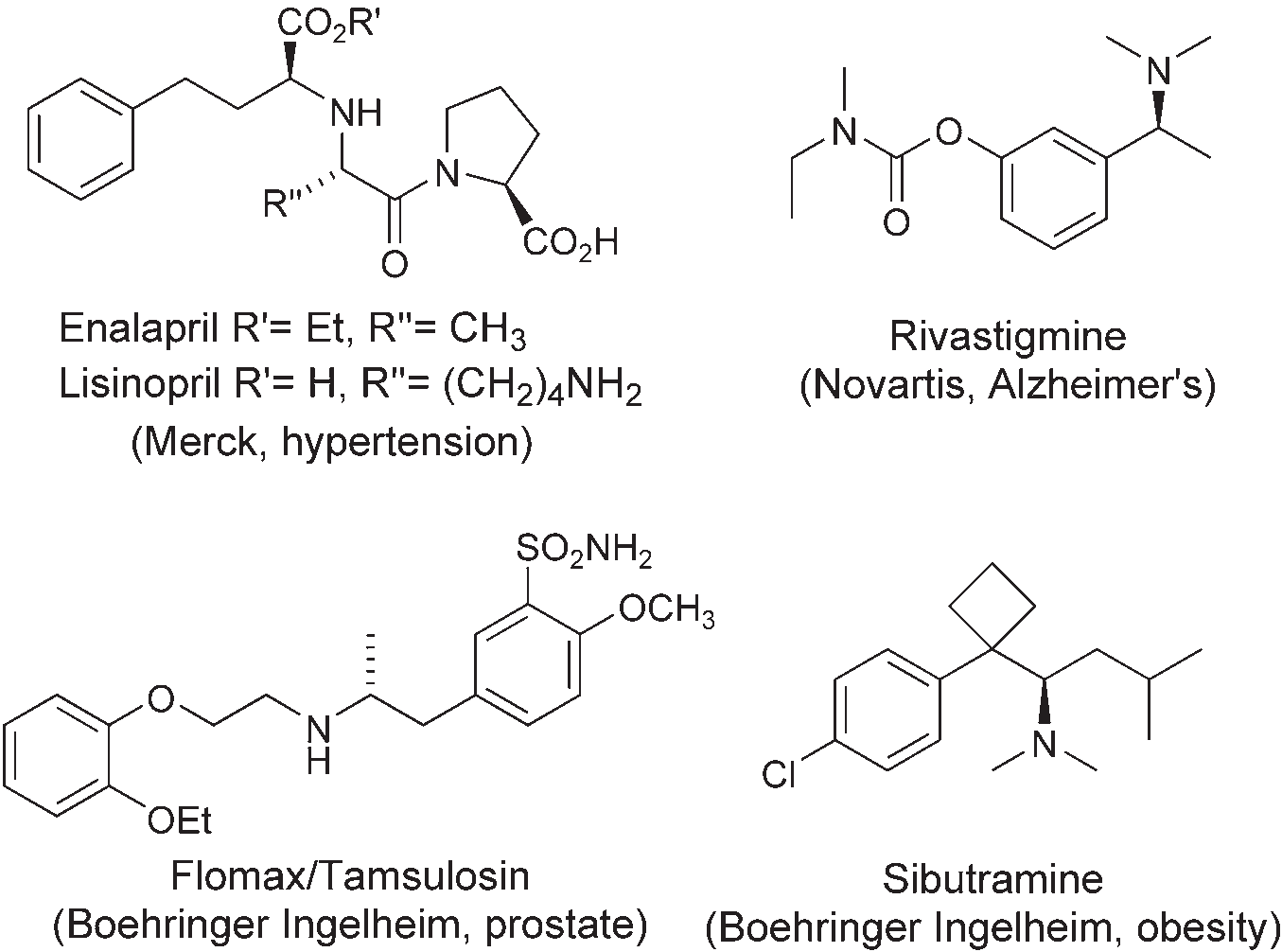
Pharmaceutical drugs containing a-chiral amine stereocenters: enalapril, lisinopril, rivastigmine, Flomax, TAMSULOSIN, sibutramine.
Related Questions and Answers
A: The main by-products of lisinopril degradation in the FeCu/NC-catalyzed electro-Fenton process include two primary aromatic compounds identified by LC-MS/MS analysis. These by-products are likely formed through the initial oxidation of lisinopril by hydroxyl radicals, followed by further degradation into smaller aromatic and aliphatic compounds. The final degradation products are short-chain linear carboxylic acids, such as oxalic acid, which are refractory intermediates in the mineralization process. The presence of these by-products indicates that the FeCu/NC catalyst effectively degrades lisinopril into simpler compounds, although complete mineralization to CO2 and H2O is not fully achieved within the tested time frame.
A: The FeCu/NC catalyst outperforms both Fe/NC and Cu/NC catalysts in the electro-Fenton process for lisinopril degradation. It achieves complete degradation of LSN at a lower dosage (0.05 g L−1) and in a shorter time compared to Fe/NC and Cu/NC. The FeCu/NC catalyst maintains high stability and low metal leaching even after multiple cycles, demonstrating superior reusability. The presence of low-valence copper in FeCu/NC promotes the Fe(III)-to-Fe(II) conversion, which is crucial for the continuous activation of H2O2 and the generation of hydroxyl radicals. This synergistic effect enhances the overall efficiency of the electro-Fenton process.
A: The FeCu/NC catalyst plays a crucial role in the electro-Fenton degradation of lisinopril by facilitating the activation of hydrogen peroxide (H2O2) to produce hydroxyl radicals (•OH), which are strong oxidizing agents capable of degrading LSN. The catalyst's core-shell structure, with a carbon layer encapsulating the FeCu metallic core, provides high stability and reduces metal leaching. The presence of low-valence copper in the catalyst promotes the continuous regeneration of Fe(II), which is essential for the Fenton's reaction. This catalyst achieves complete LSN degradation at a low dosage (0.05 g L−1) within 6 minutes at pH 3 and 75 minutes at near-neutral pH, demonstrating its high efficiency and stability.
A: Lisinopril significantly increases the whole-body metabolic rate in young flies with AD-like symptoms, but not in old flies. This suggests that lisinopril may have therapeutic potential in improving metabolic function in younger individuals with AD-like symptoms. The findings support the idea that ACE inhibitors could play a role in modulating metabolic processes in neurodegenerative diseases, potentially through mechanisms involving the renin-angiotensin system.
A: Lisinopril improves cold tolerance in Drosophila melanogaster in a genotype-, sex-, and age-specific manner. Specifically, lisinopril-treated DGRP_367 young males and DGRP_808 old males exhibited significantly faster recovery from chill-induced coma compared to untreated males. The expression of Ucp4b and Ucp4c genes, which are involved in cold-induced thermogenesis, was significantly increased in the heads of lisinopril-treated DGRP_367 young male flies. This suggests that lisinopril enhances cold tolerance through the induction of UCPs, similar to the mechanism observed in mammals.
A: The effect of lisinopril on whole-body metabolic rate depends on Ance expression in the Malpighian/renal tubules and the nervous system. Knocking down Ance in these tissues results in significant changes in metabolic rate, with the effects being sex- and age-specific. In the Malpighian tubules, Ance knockdown leads to a significant decrease in metabolic rate in both female and male flies, independent of age. In the nervous system, Ance knockdown results in a significant increase in metabolic rate in young flies, independent of sex. These findings suggest that Ance plays a critical role in modulating metabolic rate in Drosophila melanogaster through tissue-specific mechanisms.
A: Lisinopril affects the whole-body metabolic rate in Drosophila melanogaster in a genotype-, sex-, and age-dependent manner. The effects are highly context-dependent, influenced by genetic background, sex, and age. Lisinopril may increase the metabolic rate through the accumulation of a bradykinin-like peptide, which enhances cold tolerance by upregulating Ucp4b and Ucp4c genes. The effect of lisinopril also depends on the expression of Ance, the ortholog of mammalian ACE, in the Malpighian/renal tubules and the nervous system. In summary, lisinopril modulates metabolic rate through mechanisms involving both the reduction of Angiotensin II-equivalent signaling and the accumulation of bradykinin-like peptides.