Hydrogen Peroxide
CAS number: 7722-84-1
Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste. Small amounts of gaseous hydrogen peroxide occur naturally in the air. Hydrogen peroxide is unstable, decomposing readily to oxygen and water with release of heat.
Related images
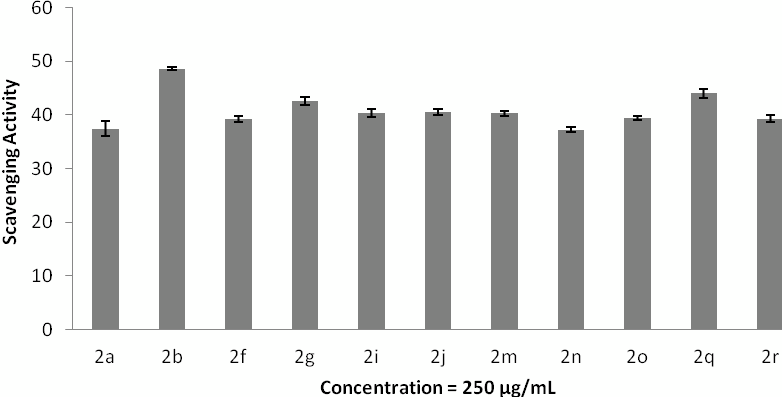
Hydrogen peroxide radical scavenging. The data represent the percentage of hydrogen peroxide radical inhibition (mean ± SD), and experiments were performed in triplicate.
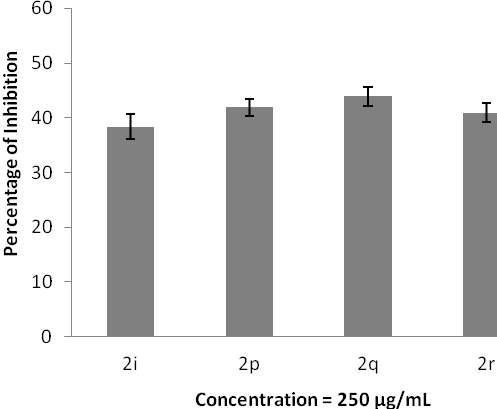
Inhibition of protein denaturation. The data represent the percentage of hydrogen peroxide radical inhibition (mean ± SD), and experiments were performed in triplicate.
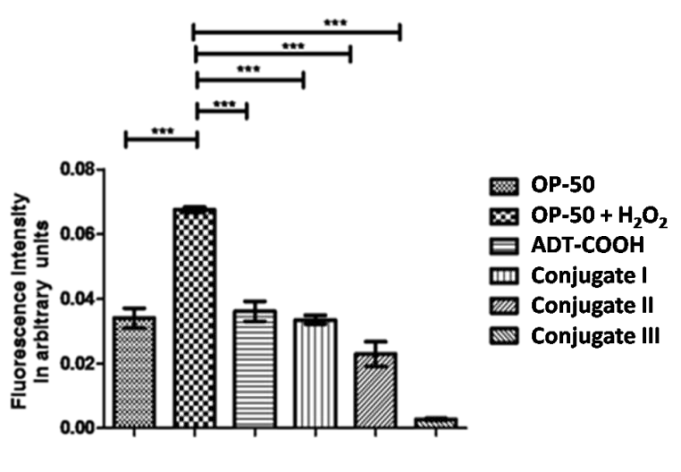
ROS estimation using DCFDA in wild type N2 strain of C. elegans on treatment with 1 mM of compound ADT-COOH, I, II and III in presence of 20 mM concentration of H2O2 (as positive control).
Related Questions and Answers
A: The study found significant mineralization of TPA and BHET using excimer lamps and hydrogen peroxide. For TPA, the KrCl lamp achieved an 80% reduction in COD after 120 minutes of treatment at a 3:1 H₂O₂/TPA mass ratio, indicating substantial mineralization. In contrast, the XeBr lamp achieved only a minor COD reduction. For BHET, the KrCl lamp resulted in a 50% decrease in COD at a 5:1 H₂O₂/BHET mass ratio, while the XeBr lamp showed practically the same COD residual value at a 4:1 ratio. These results highlight the superior mineralization efficiency of the KrCl lamp-based AOP for PET-derived pollutants.
A: Hydrogen peroxide plays a crucial role in the photodegradation process by providing hydroxyl radicals necessary for the degradation of BHET and TPA. The study found that the optimal H₂O₂/monomer mass ratios were 3:1 for TPA and 4:1 and 5:1 for BHET with XeBr and KrCl lamps, respectively. Exceeding these ratios led to reduced efficiency due to hydroxyl radical scavenging. The presence of H₂O₂ significantly enhanced the degradation rates, achieving near-complete conversion of TPA and BHET under optimal conditions.
A: Hydrogen peroxide induces different responses in NIHs and NIHv cells, which are related to their basal proliferative activities and redox states. In NIHs cells, hydrogen peroxide treatment leads to a significant decrease in proliferative activity, increased expression of senescence-associated markers (e.g., SA-β-galactosidase positivity), and changes in mitochondrial and lysosomal content/activity. These changes are indicative of the acquisition of a senescent phenotype. In NIHv cells, which are already in a senescent state, hydrogen peroxide treatment further reduces proliferative activity and increases the accumulation of lipofuscin, a marker of cellular aging. The treatment also affects the mitochondrial and lysosomal compartments, suggesting that hydrogen peroxide can modulate senescence and transformation through redox-dependent mechanisms. The study concludes that hydrogen peroxide can induce senescence in transformed cells and modulate the senescence state in already senescent cells, highlighting the complex interplay between oxidative stress, cellular senescence, and transformation.
A: The study found significant disparities in the content of hydrogen peroxide and organic peroxides in different samples. Hydrophilic samples like HPMC, MCC, and honey predominantly contained hydrogen peroxide, while more lipophilic samples like vegetable oil and milk were characterized by the presence of organic peroxides. This variation is attributed to factors such as synthesis processes, storage conditions, and sample age. The content of peroxides in these materials can influence their suitability for specific applications. For instance, high levels of hydrogen peroxide in excipients can lead to oxidation of active pharmaceutical ingredients, while organic peroxides in food products can affect shelf life and safety.
A: Differentiating between hydrogen peroxide and organic peroxides is important because they exhibit different reactivities and can have varying implications for product stability and safety. For example, hydrogen peroxide can initiate oxidation reactions that lead to degradation of active pharmaceutical ingredients, while organic peroxides can generate radicals that affect polymer stability. The ability to distinguish between these peroxides allows for better control over product quality, selection of appropriate antioxidants, and optimization of storage conditions. The differentiation is crucial for ensuring product quality, safety, and stability in both pharmaceutical and food industries.
A: The one-pot HPLC method developed in this study utilizes borinic acid for the selective reaction with hydrogen peroxide, followed by the reaction of triphenylphosphine with the remaining organic peroxides. The resulting phenol derivative and triphenylphosphine oxide are detected using HPLC with UV detection. This method allows for the simultaneous quantification of both hydrogen peroxide and organic peroxides in a single run, providing a simple, robust, and versatile tool for precise peroxide monitoring in various applications. This novel HPLC method offers a significant improvement over existing methods by enabling the differentiation of peroxide species, which is crucial for guiding excipient selection and supporting antioxidant use in formulation development.
A: Hydrogen peroxide (H2O2) acts as an oxidant, reacting with the catalyst's active sites to produce reactive oxygen species that are critical for the degradation of organic pollutants in the Fenton reaction.