Acetone
CAS number: 67-64-1
Acetone is a colorless, volatile, flammable liquid organic compound, primarily used as a solvent and in the production of various industrial and consumer products.
Related images
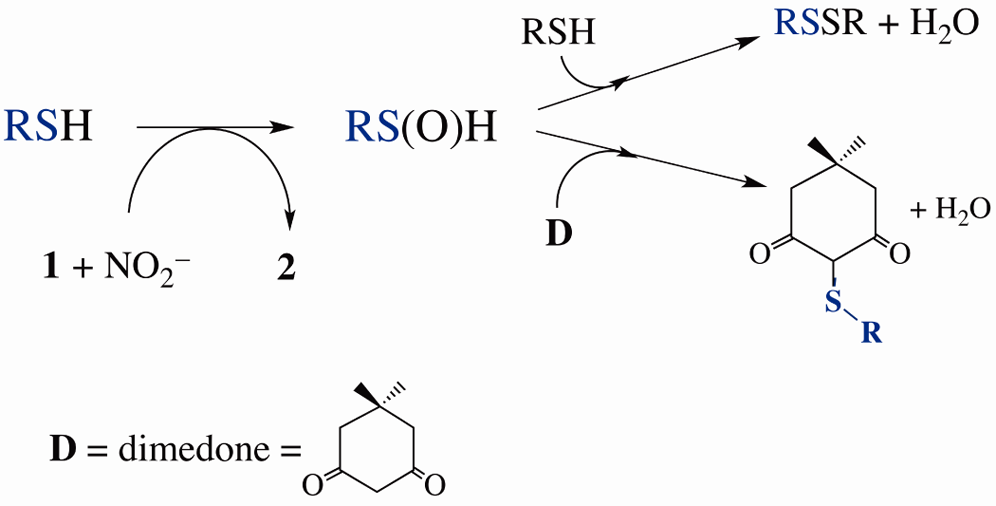
Heme-mediated OAT to RSH (CysSH or GSH) generates sulfinic acid RS(O)H, which is trapped by excess RSH to form RSSR or by dimethyl ketone (D) to form D−SR
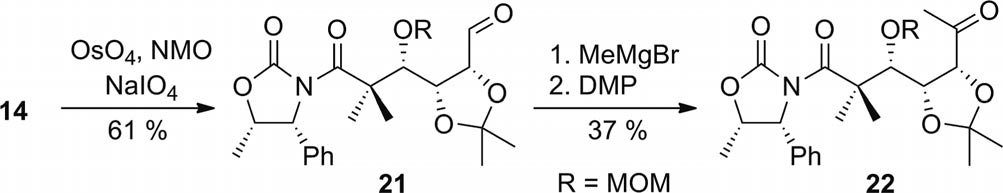
Preparation of methyl ketone 22 (NMO = N-methyl-morpholine-N-oxide; DMP = Dess–Martin periodinane).
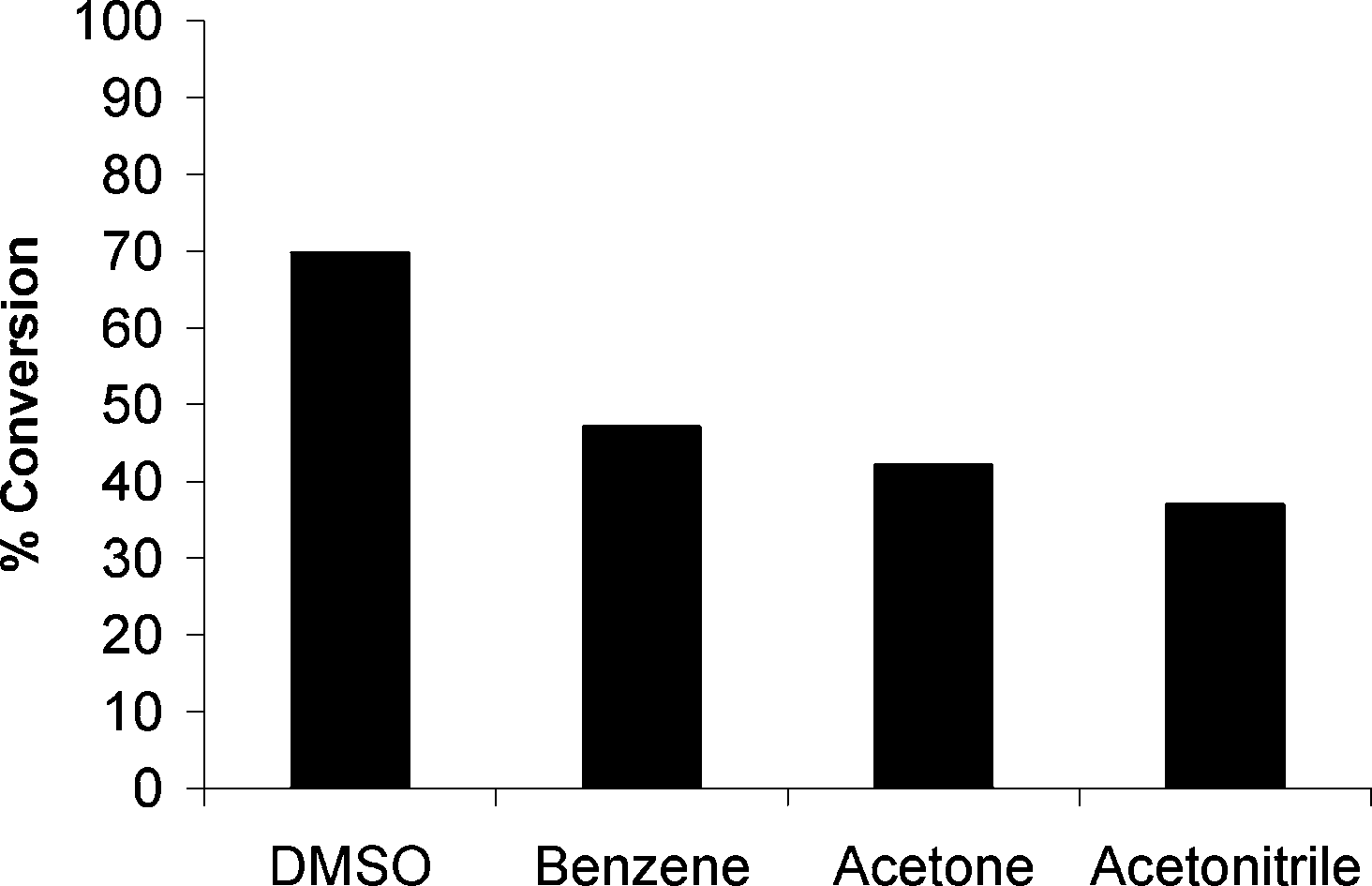
Elimination of aryl phosphonates from compound 12 was carried out in different solvents at 120 °C for 1.5 h.
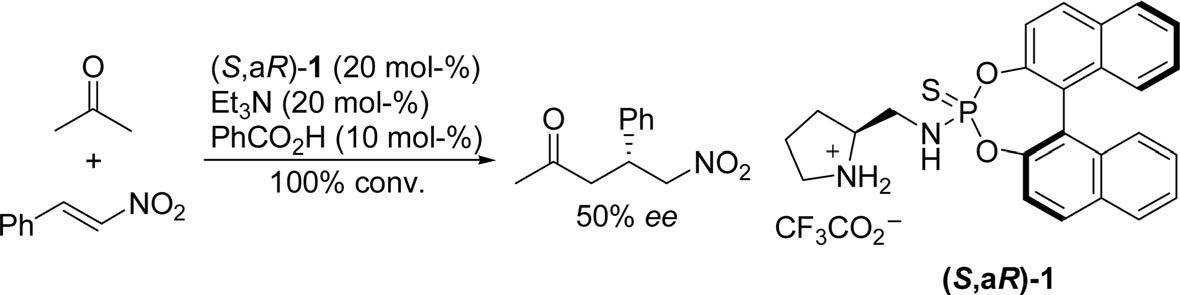
(S,aR)-1-catalyzed asymmetric Michael addition of acetone to β-nitrostyrene.
Related Questions and Answers
A: The sensing mechanism for acetone on In2O3 is not based on the total combustion of acetone. Instead, the hydroxylated In2O3 surface decomposes acetone, which leads to the consumption of hydroxyl groups and the formation of adsorbed acetates. The overall consumption of surface oxygen resulting from this process is responsible for the observed increase in conductance during gas-sensing tests.
A: Detecting acetone in breath requires complex headspace sample handling, a method where acetone can be lost during sample pretreatment. Additionally, acetone's volatility allows it to diffuse from exhaled breath into the oral cavity and dissolve in saliva, making breath analysis more challenging.
A: For acetone, the parameters are: m = 2.7447, σ/Å = 3.2742, ε/k_B/K = 232.99, μ/D = 2.88, and 10²⁰κ_DGT/Jm⁵mol⁻² = 10.944. For carbon dioxide, the parameters are: m = 1.5131, σ/Å = 3.1869, ε/k_B/K = 163.33, Q/DA = 4.4, and 10²⁰κ_DGT/Jm⁵mol⁻² = 2.4197.
A: For acetone, the force field by Windmann et al. was utilized. It comprises four Lennard-Jones sites representing two CH₃ groups, the carbonyl C atom, and the O atom, along with one point dipole and one point quadrupole to account for the molecule's polarity. For carbon dioxide, the force field by Merker et al. was used, consisting of three Lennard-Jones sites for each atom and a point quadrupole at the center of mass. Both molecular models were considered rigid.
A: Acetone is a biomarker of diabetes mellitus. People with diabetes tend to have higher concentrations of acetone in their breath compared to healthy individuals. The range of exhaled acetone in healthy people is 0.2-1.8 ppm, while for people with diabetes, it is 1.25-2.5 ppm, or even up to 25 ppm for type 1 diabetes.