Methanol
CAS number: 67-56-1
Methanol appears as a colorless fairly volatile liquid with a faintly sweet pungent odor like that of ethyl alcohol. Completely mixes with water. The vapors are slightly heavier than air and may travel some distance to a source of ignition and flash back. Any accumulation of vapors in confined spaces, such as buildings or sewers, may explode if ignited. Used to make chemicals, to remove water from automotive and aviation fuels, as a solvent for paints and plastics, and as an ingredient in a wide variety of products.
Related images
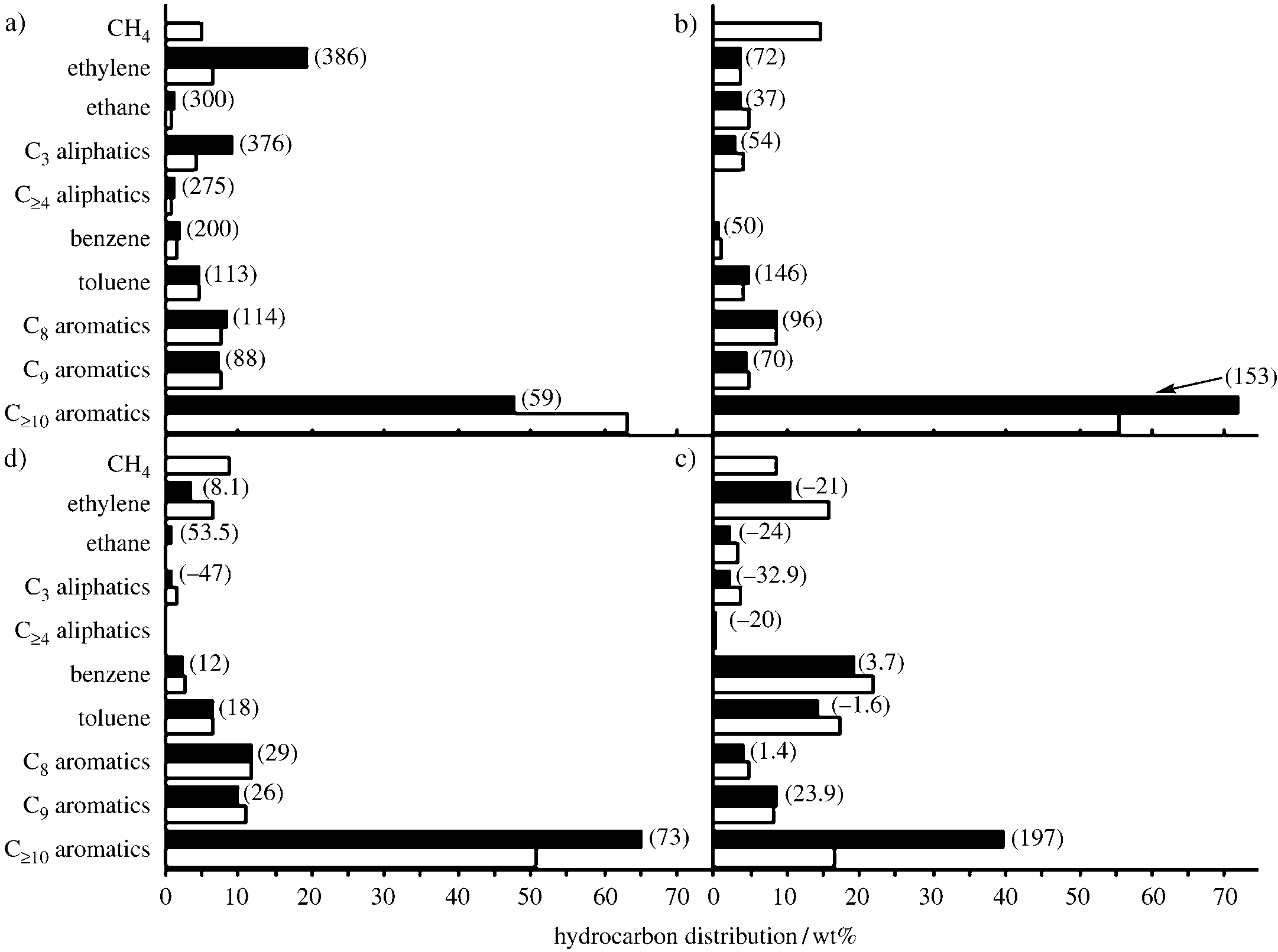
Distribution of hydrocarbons formed in the aromatization of a) methanol over H-GaAl-ZSM-5 at 5508C, b) methanol over Mo-Zn/H-ZSM-5 at 5008C, c) ethanol over H-GaAl-ZSM-5 at 6258C, and d) dimethyl ether (DME) over H-GaAl-ZSM-5 at 5508C in the presence (solid bars) and absence (open bars) of methane.
Related Questions and Answers
No related questions yet