Dimethyl Sulfoxide
CAS number: 67-68-5
Dimethyl sulfoxide is a 2-carbon sulfoxide in which the sulfur atom has two methyl substituents. It has a role as a polar aprotic solvent, a radical scavenger, a non-narcotic analgesic, an antidote, a MRI contrast agent, an Escherichia coli metabolite, a geroprotector and an alkylating agent. It is a sulfoxide and a volatile organic compound.
Related images
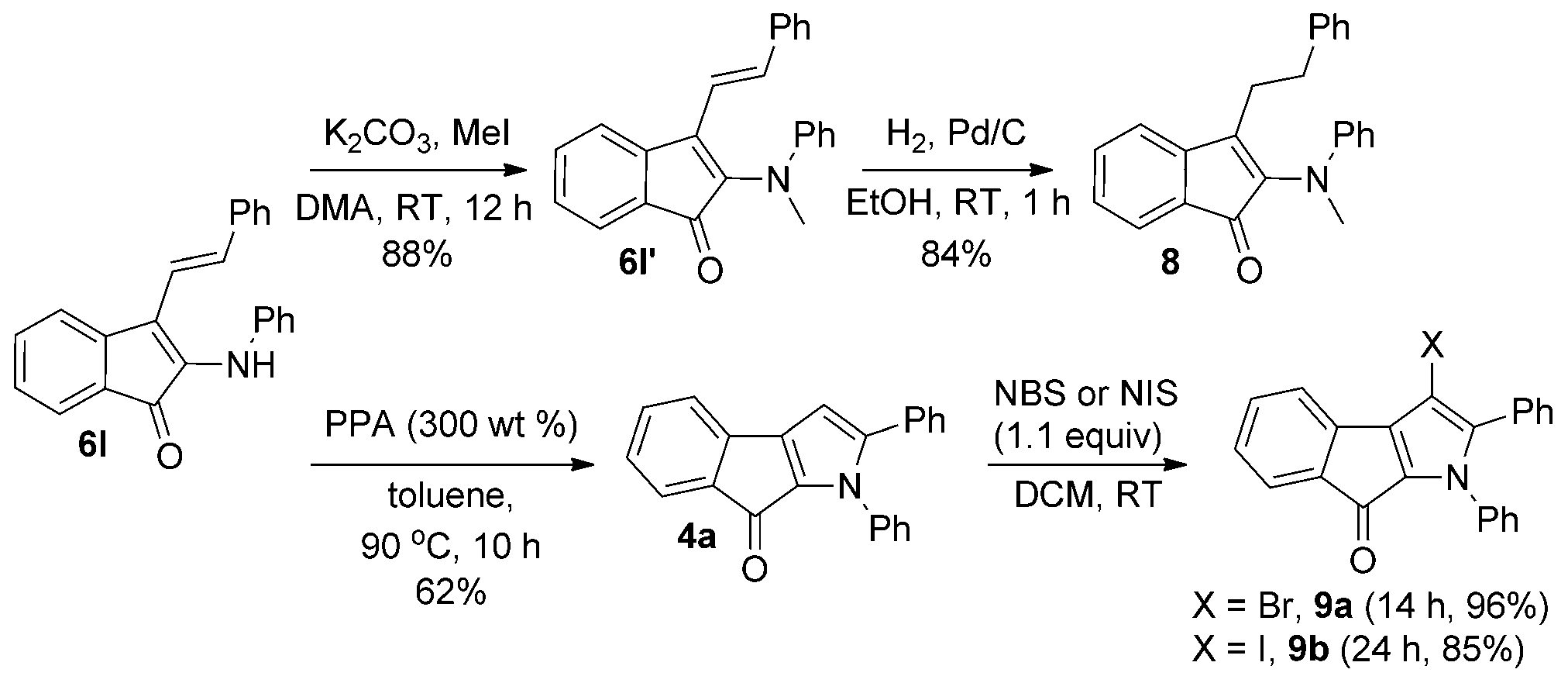
Chemical transformations. DmS=dimethylsulfoxide, NBS=N-bromosuccinimide, NIS=N-iodosuccinimide, PPA=poly-phosphoric acid.
Related Questions and Answers
No related questions yet