N,N-Dimethylaniline
CAS number: 121-69-7
N,N-dimethylaniline is a tertiary amine that is aniline in which the amino hydrogens are replaced by two methyl groups. It is a tertiary amine and a dimethylaniline.
Related images
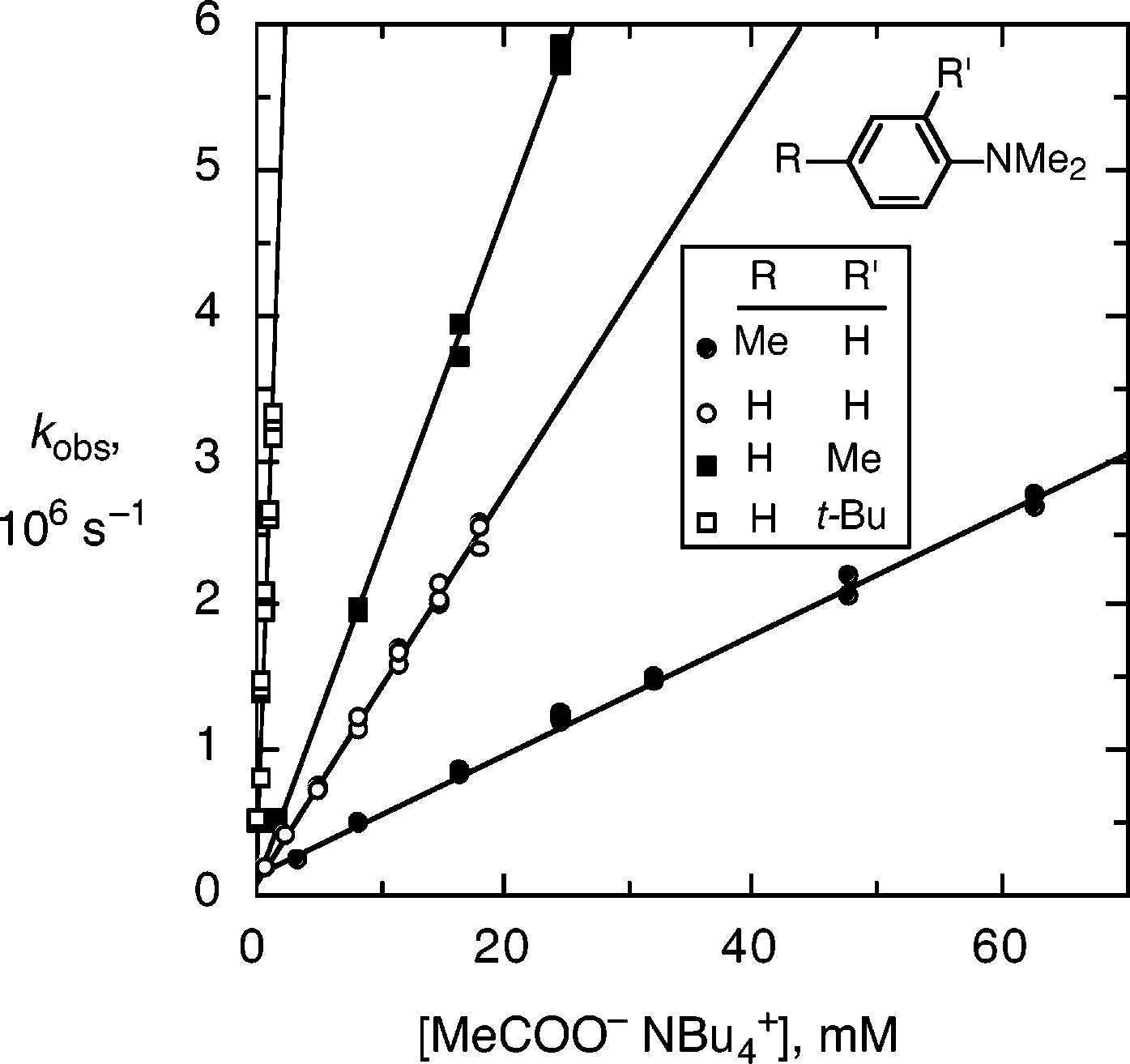
Plots of observed rate of pseudo-first-order decay, kobs, versus concentration of tetrabutylammonium acetate for deprotonation of the radical cations of (open circles) N,N-dimethylaniline, 1c, and N,N,-dimethylaniline with (filled circles) a 4-methyl substituent, 1b, (filled squares) a 2-methyl substituent, 2, and (open squares) a 2-tert-butyl substituent, 3, in acetonitrile with 0.5 m water and 0.5 m tetramethylammonium perchlorate, at room temperature.
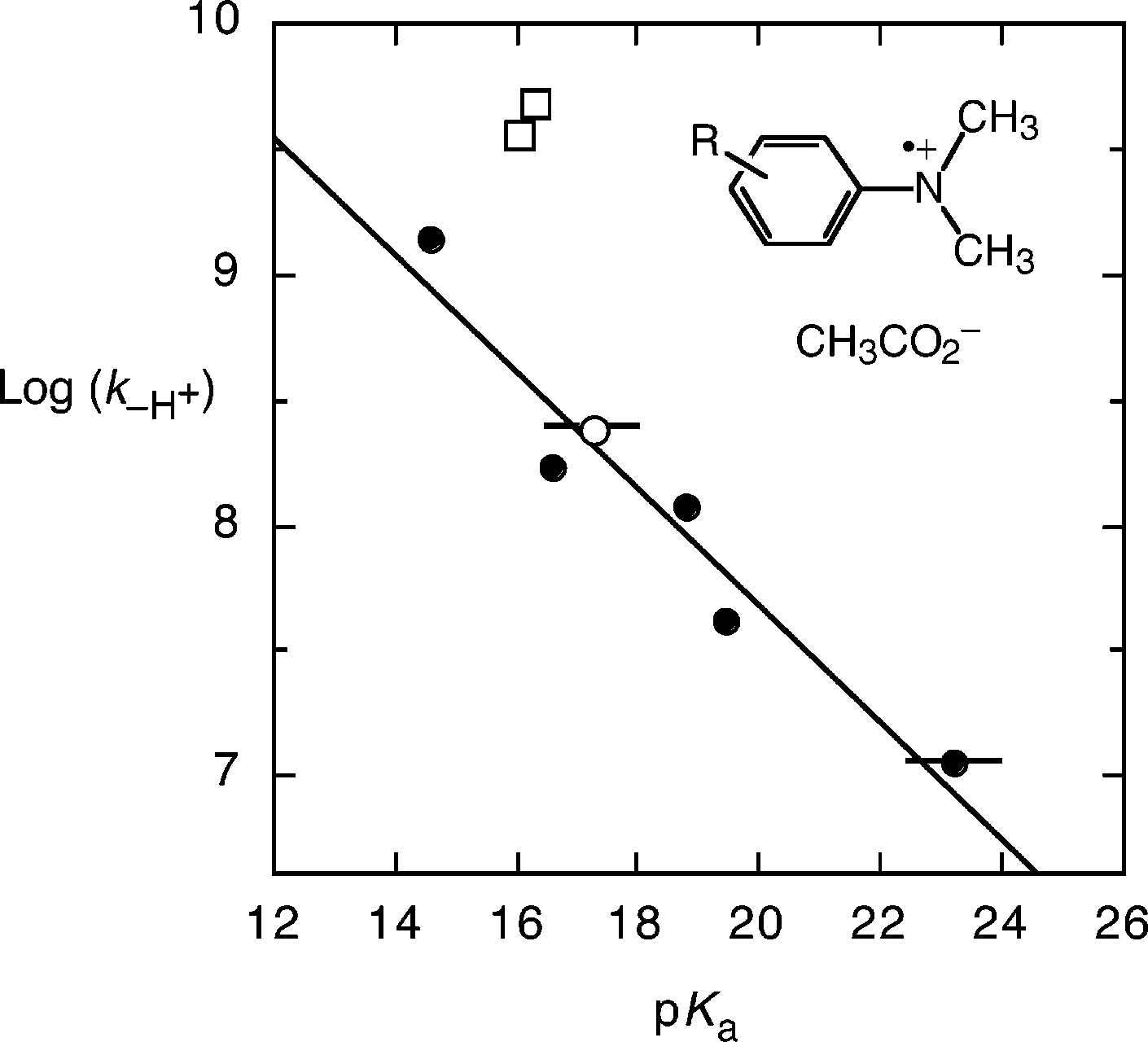
Brønsted plot of the logarithm of the bimolecular rate constant for deprotonation of the radical cations of N,N-dimethylaniline derivatives: (closed circles) 4-substituted-N,N-dimethylanilines, 1a-1e; (open circle) N,N,2-trimethylaniline, 2; (open squares) 2-tert-butyl-N,N-dimethylaniline, 3; and N,N,2,6-tetramethylaniline, 4.
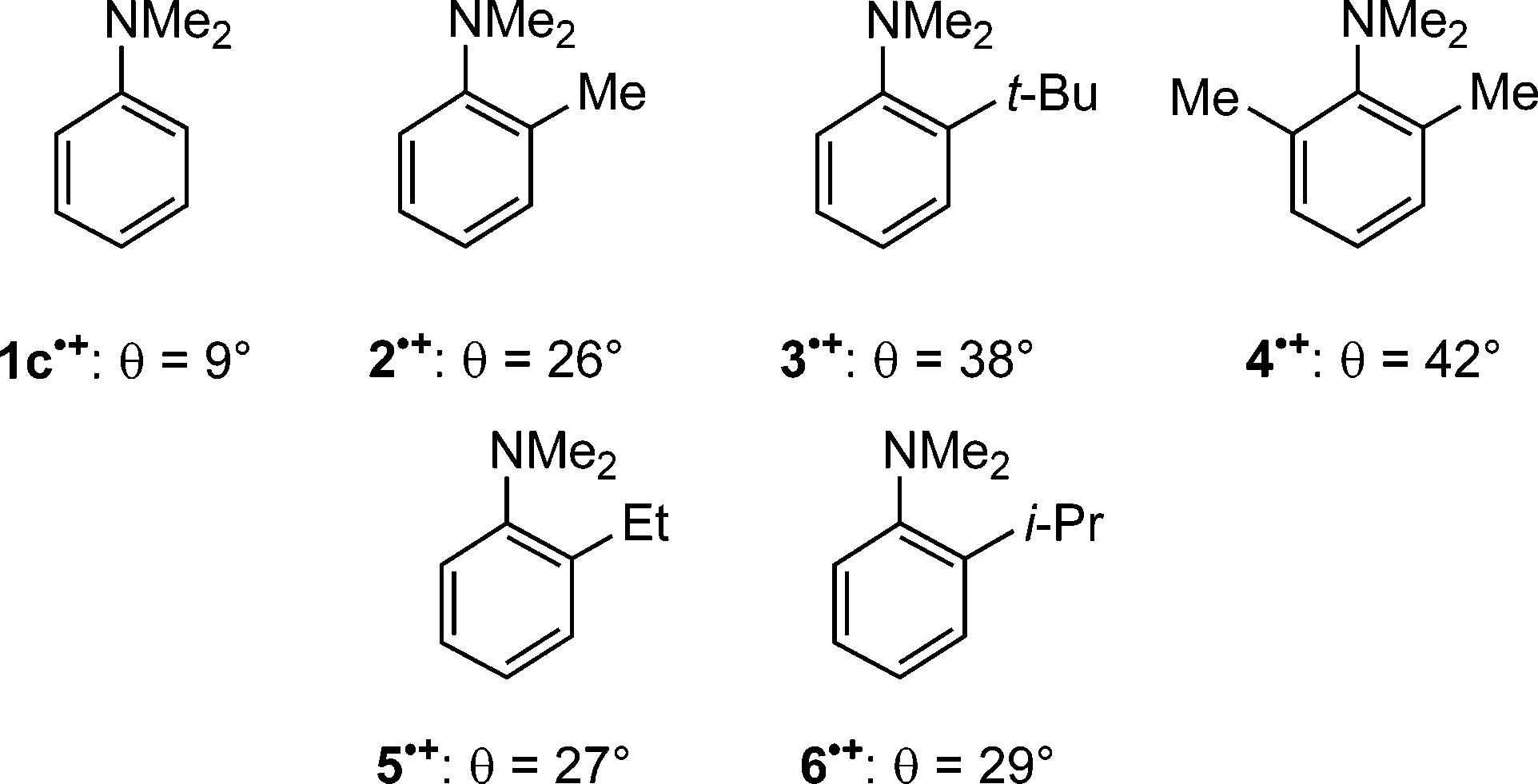
Structures of Sterically Twisted N,N-Dimethylaniline Radical Cations, and the Dihedral Angles between the Planes Containing the Aromatic Ring and the Amino Nitrogen, θ.
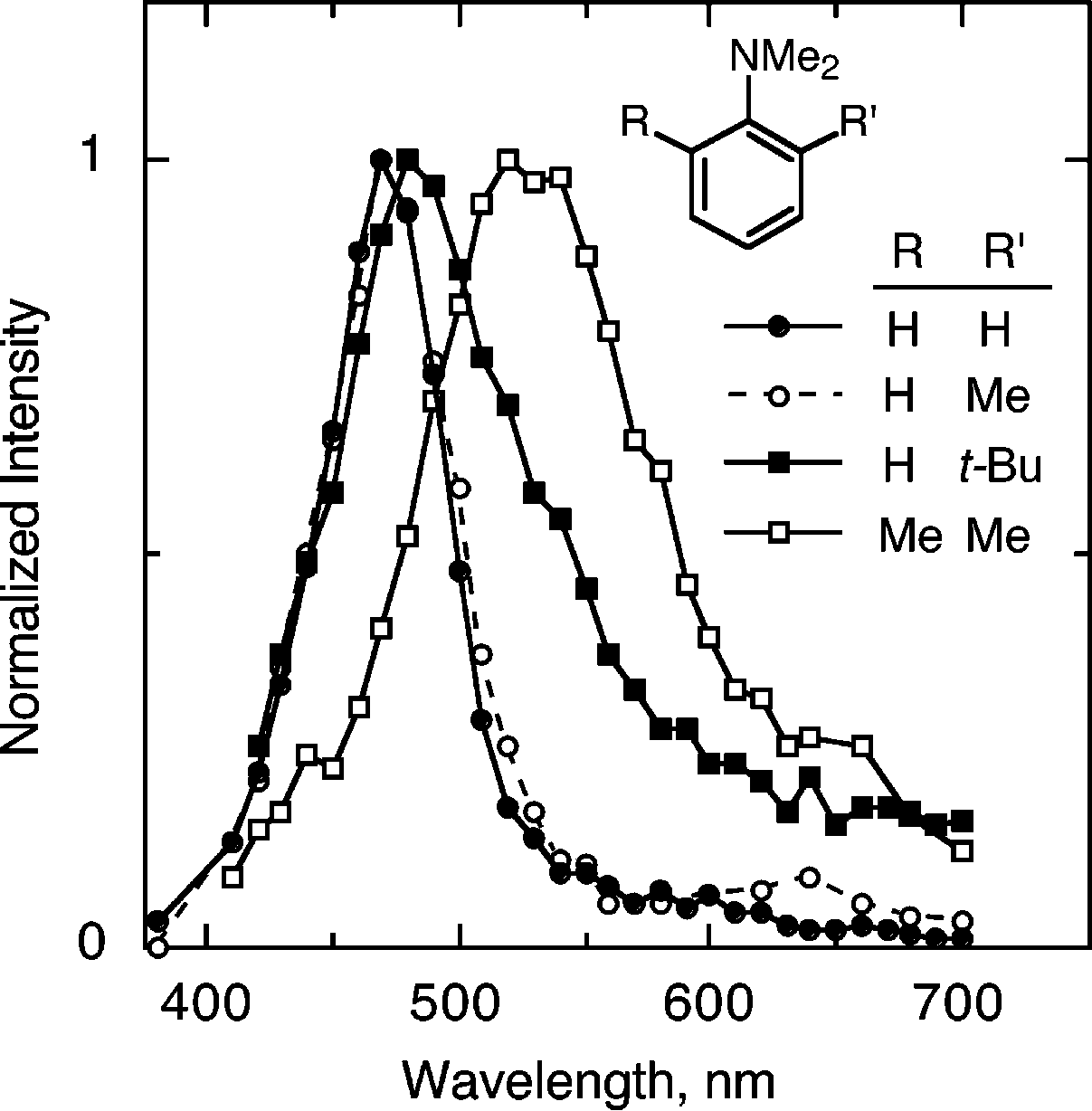
Absorption spectra of the radical cations of (closed circles) N,N-dimethylaniline, 1c, and N,N,-dimethylaniline with (open circles) a 2-methyl substituent, 2, (closed squares) a 2-tert-butyl substituent, 3, and (open squares) 2,6-dimethyl substituents, 4, in acetonitrile containing 0.5 m water and 0.5 m tetrabutylammonium perchlorate, at room temperature.
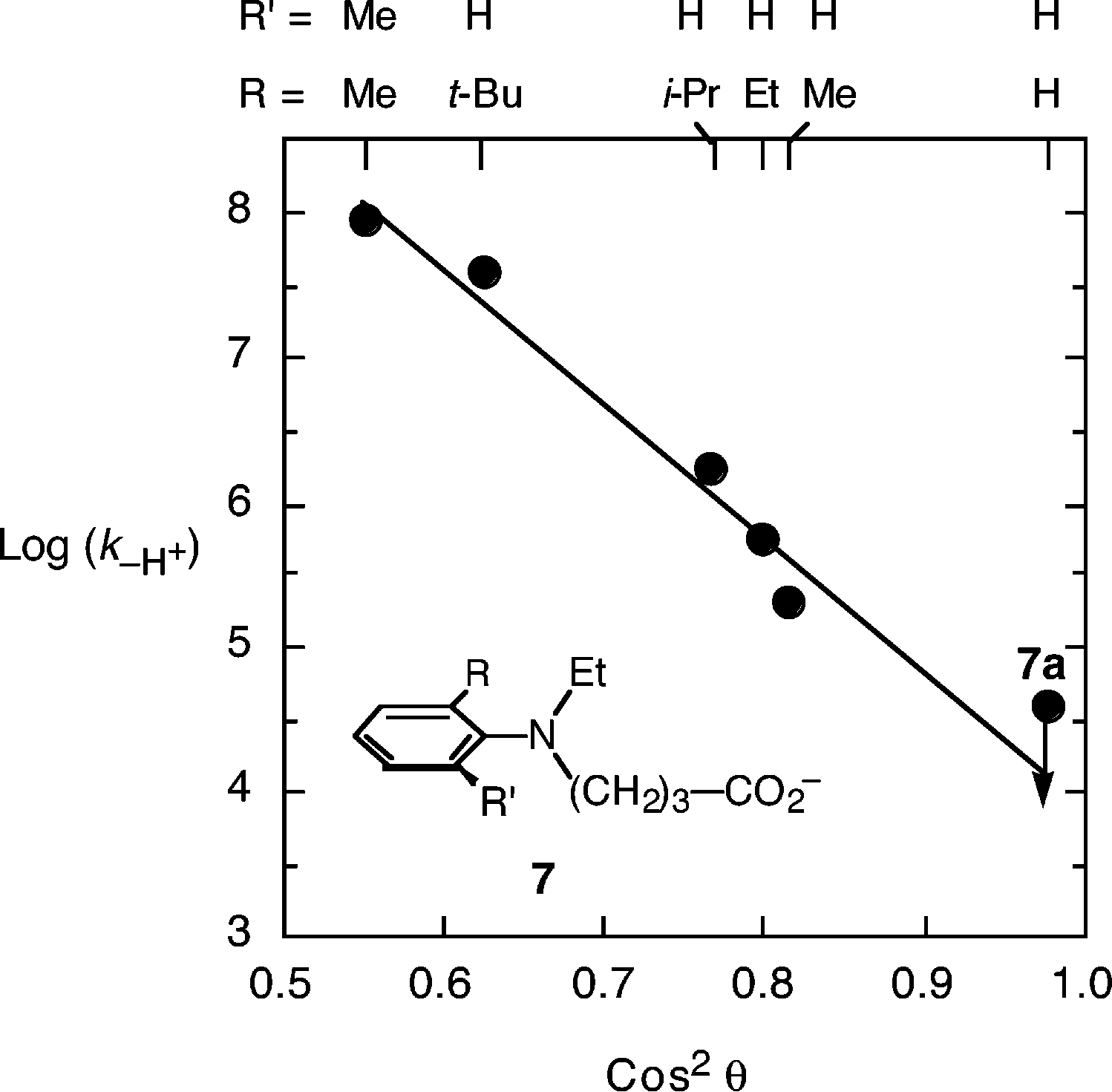
Plot of the logarithm of the rate constant for unimolecular deprotonation (log(k-H )) versus the cosine squared of the dihedral angle θ, for the simple dimethylanilines, assumed to be appropriate for the intramolecular deprotonating anilines here.
Related Questions and Answers
No related questions yet