Triethylamine
CAS number: 121-44-8
Et3N is the chemical formula for triethylamine, a colorless, flammable liquid with a strong ammonia-like odor.
Related images
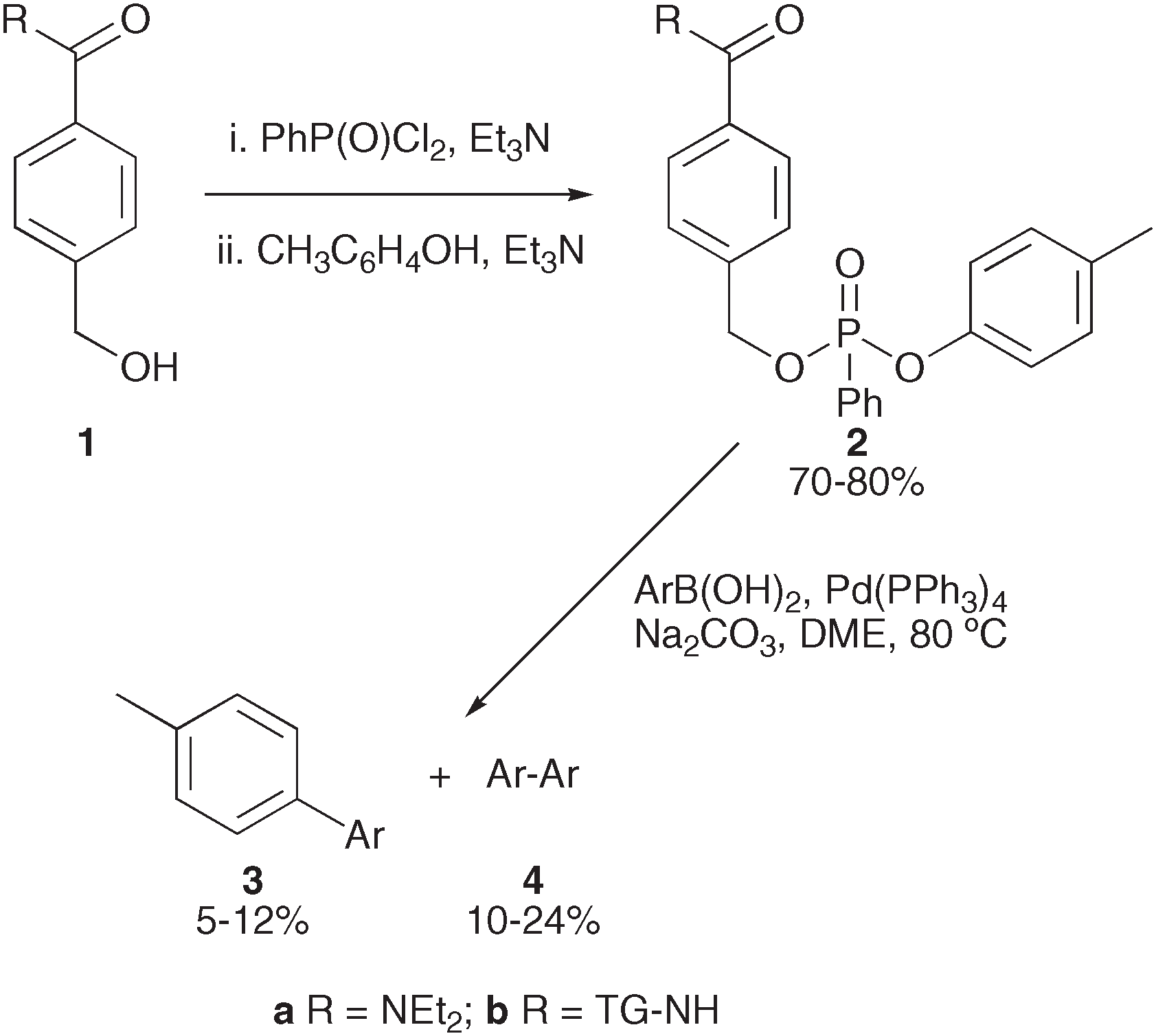
Benzyl alcohol 1a was reacted with 1.25 equivalents of phenylphosphonyl dichloride (PPDC; PhP(O)Cl2) in the presence of triethylamine (Et3N) to afford a mixture of mono- and bis-phosphates. The monosubstituted product was reacted directly with p-cresol without purification to afford the desired phenylphosphonate 2a.
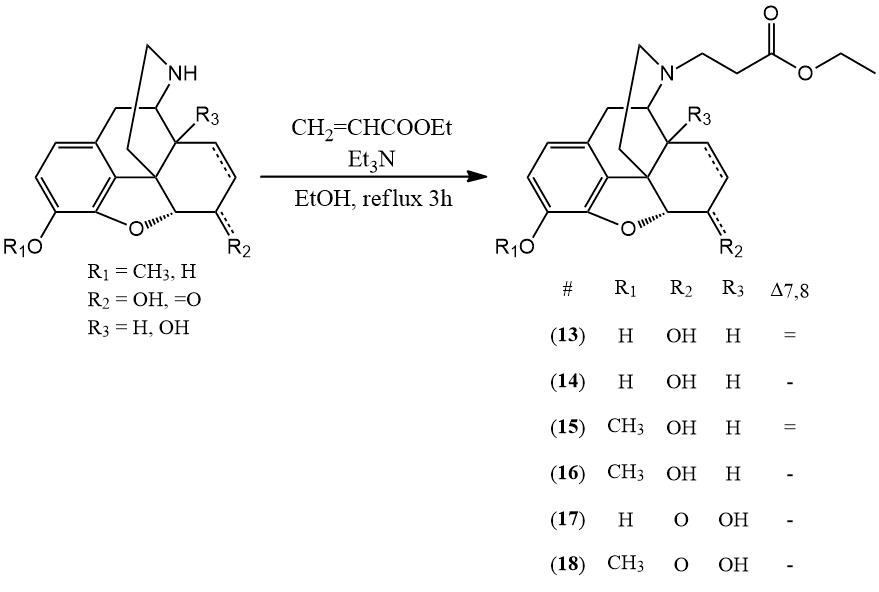
N-alkylation of nor-compounds: ethyl acrylate, triethylamine, ethanol, refl. 3 h.
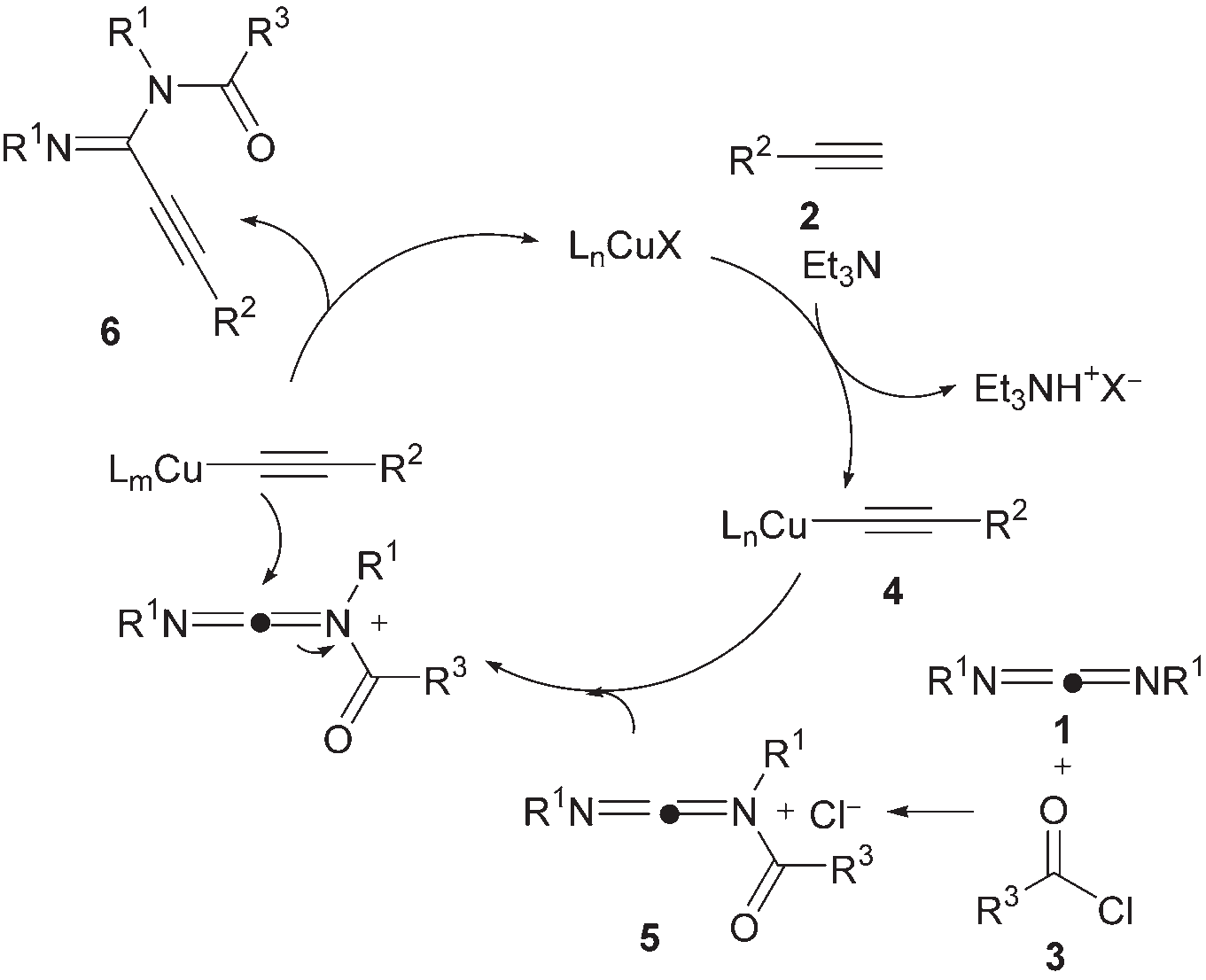
Carbodiimide 1 reacts with acyl chloride 3 to generate N-acylimide salt 5. Alkyne 2 is immediately converted to acetylide copper 4 in the presence of triethylamine, and 1 equivalent of triethylamine acid salt is released. Subsequently, 4 undergoes nucleophilic attack on 5 to obtain the target product 6, and the copper catalyst is released, completing the catalytic cycle.
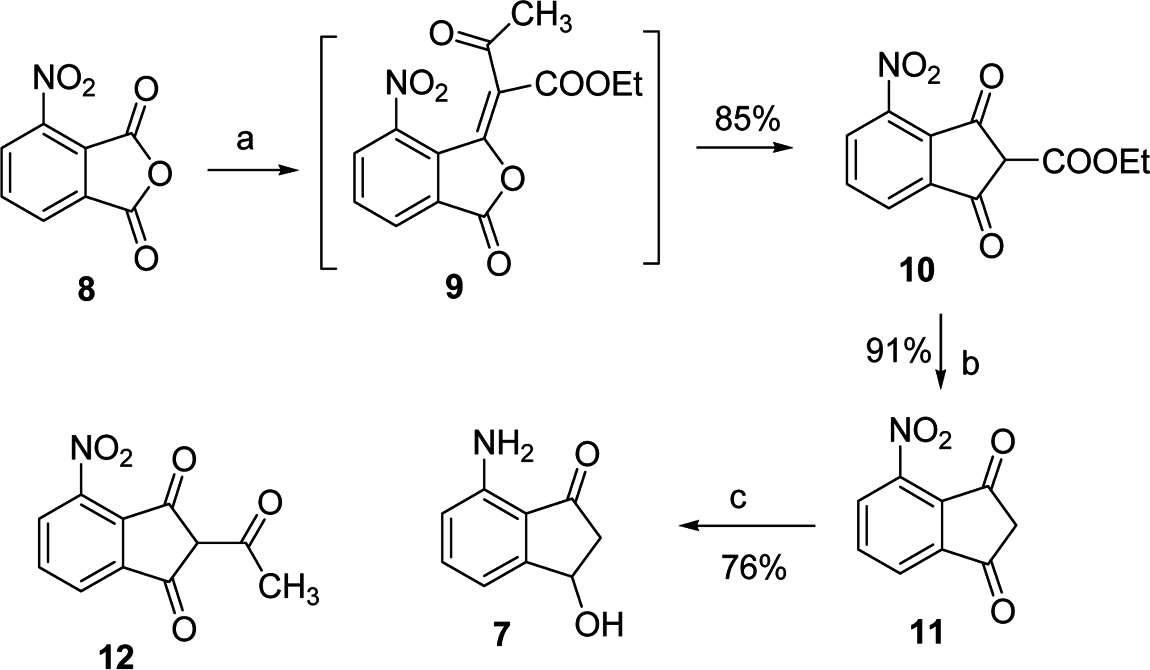
Reagents and conditions: (a) ethyl acetoacetate, acetic anhydride, triethylamine, dichloromethane, room temperature, 30 min; (b) trifluroacetic, acetonitrile, room temperature, 45 min; (c) 40 psi H2, 10% Pd=C, methanol, 24 h.
Plots of on-line pressure monitored versus time for reactions using CH3CN/H2O/Et3N (2:8:0.2) as solvent mixture under irradiation at l=447 nm.
Related Questions and Answers
No related questions yet