Hydroxylamine
CAS number: 7803-49-8
Hydroxylamine is the simplest hydroxylamine, consisting of ammonia bearing a hydroxy substituent. It is an intermediate in the biological nitrification by microbes like bacteria. It has a role as a nitric oxide donor, an EC 1.1.3.13 (alcohol oxidase) inhibitor, a nucleophilic reagent, an EC 4.2.1.22 (cystathionine beta-synthase) inhibitor, an EC 4.3.1.10 (serine-sulfate ammonia-lyase) inhibitor, a bacterial xenobiotic metabolite and an algal metabolite. It is a conjugate acid of a hydroxyazanide and an aminooxidanide. It derives from a hydride of an ammonia.
Related images
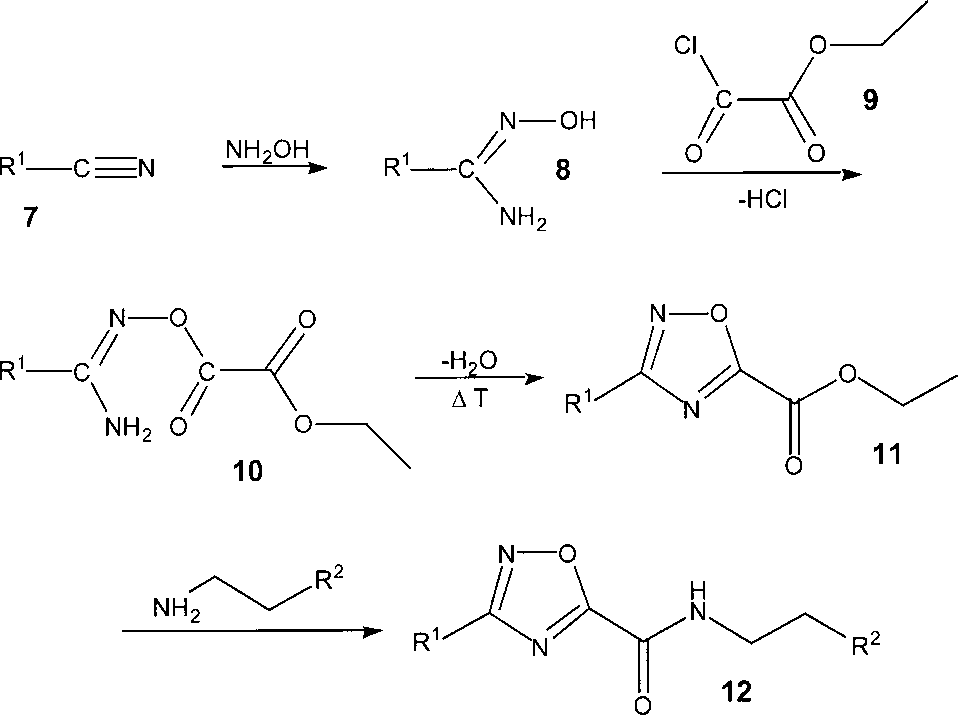
Synthesis of 1,2,4-oxadiazole-5-carboxamides 12 from the nitriles 7 and hydroxylamine.
Related Questions and Answers
A: The initial concentration of hydroxylamine (HA) in the feed phase significantly affects the separation efficiency and purity in the PIM-ED process. As the initial concentration of HA increased from 0.05 mol·L−1 to 0.25 mol·L−1, the flux and purity of HA increased due to enhanced contact opportunities between HA and the carriers, promoting the formation of ion-carrier complexes. However, when the concentration further increased to 0.51 mol·L−1, the purity of HA decreased from 99.4% to 79.8%. This reduction is attributed to the presence of metal ions from the carbon electrode, which accelerated the decomposition of HA at higher concentrations. Therefore, controlling the initial concentration of HA is crucial for achieving high-purity HA in the PIM-ED process.
A: Electrodialysis (ED) plays a crucial role in the PIM-ED process by efficiently and continuously providing the proton gradient required for the transport of hydroxylamine (HA) without introducing other ions. The ED process uses an external electric field to generate protons and hydroxide ions, which are transported to the stripping phase and feed phase, respectively. This maintains the proton gradient between the two phases, enhancing the driving force for HA transport. The study found that the ED process significantly increased the permeability coefficient of HA, with optimal results achieved at a current density of 10 mA·cm−2. The ED process thus enables the selective separation of HA from metal ions and inorganic acid ions, achieving high-purity HA production.
A: The proton gradient serves as the driving force for transporting hydroxylamine (HA) in the PIM process. HA reacts with the carbonyl groups of the carrier (TTA) in the membrane, releasing protons. To maintain the chemical equilibrium of this reaction, protons need to be transported from the stripping phase to the feed phase. This proton gradient between the two phases provides the necessary driving force for the transport of HA. The study found that increasing the proton gradient (e.g., by adjusting the pH of the feed phase) enhances the permeability coefficient and extraction efficiency of HA. For instance, when the initial pH of the feed phase was increased from 1.0 to 7.3, the permeability coefficient and extraction efficiency of HA improved significantly.
A: The study presents a method using a polymer inclusion membrane (PIM) coupled with electrodialysis (ED) to separate hydroxylamine (HA) from metal ions. The PIM, containing thenoyltrifluoroacetone (TTA) as a carrier, selectively transports HA driven by a proton gradient. The ED process continuously provides this proton gradient without introducing other ions. Under optimal conditions, the separation factors for NH2OH(I)/Na(I) and NH2OH(I)/K(I) were 30.81 and 35.11, respectively, and the purity of HA reached 99.4%. This PIM-ED process effectively produces high-purity HA by controlling metal ion concentrations and pH.
A: The presence of hydroxylamine affects the calculation of nitrogen gas selectivity because it challenges the assumption that only nitrate, nitrite, and ammonium are relevant products. Ignoring hydroxylamine leads to erroneous mass balance and nitrogen gas selectivity calculations. The study emphasizes the need to account for hydroxylamine in future studies and applications.
A: The formation of hydroxylamine during nitrate and nitrite reduction poses a major setback for drinking water purification applications due to its toxicity. It is crucial to consider hydroxylamine in the catalyst and process design to ensure safe drinking water.
A: Detecting hydroxylamine is significant because it provides a more accurate understanding of the reaction mechanism and challenges the assumption that only nitrite, ammonia, and nitrogen gas are formed. It also highlights the need to account for this intermediate in the mass balance and in the design of catalysts for drinking water purification.
A: Hydroxylamine is an essential intermediate in the reduction of nitrate and nitrite, challenging the assumption of nitrogen gas selectivity and indicating that the reaction mechanism needs to be reevaluated. Its presence also poses a setback for the application of catalytic nitrate and nitrite reduction in drinking water purification due to its toxicity.