Silver Chloride
CAS number: 7783-90-6
Silver chloride is a chloride of silver that occurs naturally as the mineral chlorargyrite. It is used to make photographic paper and pottery glazes. It is also found in stained glass colorants, bandages, and other wound healing products, and may be used as an antidote to mercury poisoning. Silver is a metallic element with the chemical symbol Ag and atomic number 47. It occurs naturally in its pure, free form, as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. (L808, L809, L816)
Related images
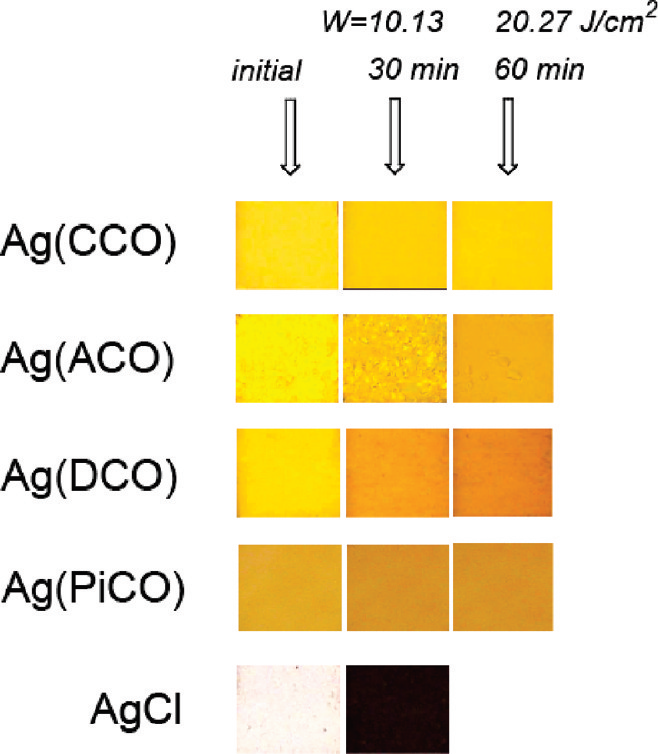
Resistance of synthesized solid silver(I) cyanoximates Ag-(CCO), Ag(ACO), Ag(DCO), and Ag(PiCO) toward high-energy UV-radiation at 254 nm in comparison to conventional light-sensitive silver chloride.
Related Questions and Answers
A: Gamma radiation affects the corrosion behavior of copper by increasing its open circuit potential (OCP) due to the oxidizing power of radiolysis species. The study shows that the OCP of copper under radiation is statistically higher than that of non-irradiated samples. Potentiodynamic polarization measurements indicate no significant difference in corrosion potential (Ecorr) and corrosion current density (jcorr) between irradiated and non-irradiated copper samples. However, an observable difference in the anodic branch suggests the possible formation of a passive film under radiation. The Ag/AgCl QREs are reliable for these measurements, confirming their suitability for in-situ electrochemical corrosion studies under gamma radiation.
A: The AgCl coating is essential for the stability of Ag/AgCl QREs under gamma radiation. The study demonstrates that the AgCl coating remains stable, with no significant changes in its chemical composition after 33 days of radiation exposure. The morphology of the AgCl crystals changes, with grain growth observed only in irradiated samples, indicating radiation-enhanced diffusion or defect generation. Despite these changes, the QREs maintain their stability, confirming the reliability of AgCl coatings for electrochemical measurements under gamma radiation.
A: The study shows that Ag/AgCl QREs remain stable under gamma radiation exposure for at least 33 days. The open circuit potential (OCP) measurements indicate no significant difference between irradiated and non-irradiated QREs. Additionally, in-situ cyclic voltammetry measurements using an internal reference (ferrocenemethanol) confirm the stability of QREs for up to 48 hours under radiation. The stability is attributed to the AgCl coating, which remains intact and does not significantly alter the electrode's potential under the tested conditions.
A: Ag/AgCl QREs are used to provide reliable and stable measurements of electrochemical corrosion processes under gamma radiation. They enable precise, low-volume electrochemical measurements and are suitable for monitoring corrosion in confined environments. The study demonstrates that these QREs are stable for at least 33 days under gamma radiation exposure, making them a viable alternative to mercury-based electrodes for in-situ electrochemical corrosion measurements in nuclear-related applications.
A: The Ag/AgCl nanoparticles synthesized with different sugar ligands showed promising antibacterial activity against multidrug-resistant Escherichia coli species. LPSA and LSA synthesized formulations exhibited better antibacterial activity compared to G5AS and LMA synthesized ones, with minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) in the μg/mL range. The presence of L-Arginine enhanced the antibacterial activity, independent of the sugar ligand chemistry and nanoparticle morphology and size.
A: The presence of chloride ions significantly affects the interaction between L-Arginine and silver ions. UV-Vis studies show that the introduction of chloride ions alters the reaction kinetics and the morphology of the nanoparticles. For example, the presence of chloride ions suppresses the formation of certain IR bands related to the interaction between L-Arginine and silver ions, indicating a change in the surface chemistry of the nanoparticles. The chloride ions also influence the formation of Ag/AgCl nanoparticles by altering the interaction between sugar ligands and silver ions.
A: The presence of L-Arginine significantly affects the synthesis and properties of Ag/AgCl nanoparticles. L-Arginine interacts with silver ions and influences the interaction between sugar ligands and silver ions, altering the morphology, size, and surface chemistry of the nanoparticles. For example, LMA and G5AS ligands result in sheet-like Ag/AgCl nanoparticles, while LSA and LPSA ligands cause the formation of anisotropic and film-like nanoparticles. The introduction of L-Arginine enhances the antibacterial activity of the nanoparticles against multidrug-resistant Escherichia coli species, independent of the sugar ligand chemistry and nanoparticle morphology and size.