Camptothecin
CAS number: 7689-03-4
Camptothecin is an alkaloid isolated from the stem wood of the Chinese tree, Camptotheca acuminata. This compound selectively inhibits the nuclear enzyme DNA topoisomerase, type I. Several semisynthetic analogs of camptothecin have demonstrated antitumor activity.
Related images
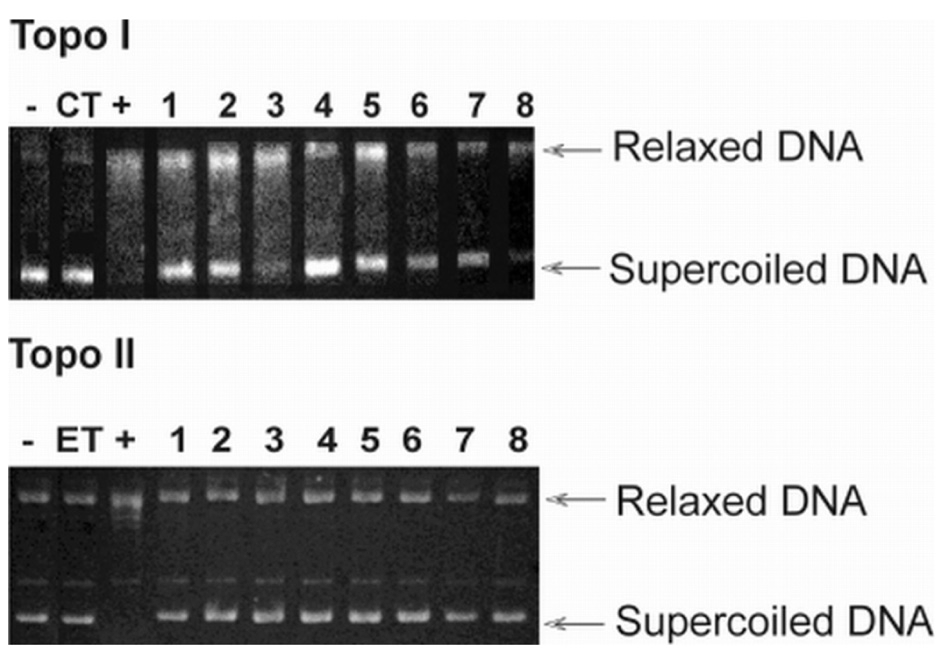
Inhibition of topoisomerase by compounds 1-8. Native pBR322 plasmid DNA (-lane) was incubated with 4 units of topoisomerase I (Topo I) and II (Topo II) without (+lane), with control (CT-camptothecin or ET-etoposide), or with drug (lanes 1-8). DNA was analyzed by 1% agarose gel electrophoresis. The gel was stained with ethidium bromide and photographed under UV light.
Related Questions and Answers
A: Camptothecin adheres to Lipinski's rule of five, with a logP value of 2.03, a molecular weight of 348.36 Daltons, one hydrogen bond donor, and six hydrogen bond acceptors, indicating favorable drug-likeness and bioavailability. Compared to neratinib, a commercially used breast cancer drug that violates two of Lipinski's rules (logP of 5.33 and molecular weight of 557.05 Daltons), camptothecin demonstrates superior compliance with drug-likeness criteria. Additionally, camptothecin is moderately soluble in water, has high gastrointestinal absorption, and does not inhibit cytochrome P450 enzymes, which is crucial for proper metabolism and elimination. However, its low solubility remains a significant challenge, which could be addressed through nanotechnology-based formulations to enhance its therapeutic efficacy.
A: Camptothecin exhibits a binding energy of −6.00 kcal/mol and a dissociation constant (Kd) of 40.01 µM with EGFR, which is less favorable compared to its interaction with HER2. The interactions between camptothecin and EGFR are primarily mediated by hydrogen bonds, particularly with Tyrosine 830, and hydrophobic interactions involving Leucine 694, Leucine 820, and Valine 702. While these interactions stabilize the camptothecin-EGFR complex, the overall binding affinity is weaker than that observed with HER2, suggesting that camptothecin may have a higher selectivity for HER2. This differential binding behavior indicates that camptothecin could potentially offer a targeted approach to treat HER2-positive breast cancer while minimizing off-target effects on EGFR.
A: Camptothecin demonstrates a strong binding affinity for HER2, with a binding energy of −8.03 kcal/mol and a dissociation constant (Kd) of 1.30 µM, which is more favorable than that of neratinib, a known HER2 inhibitor. The molecular interactions between camptothecin and HER2 are predominantly hydrophobic, involving key amino acids such as Leu726, Leu852, and Ala751, with additional hydrogen bonds forming with Lys753 and Asp863. Molecular dynamics simulations show that the camptothecin-HER2 complex remains stable over 100 nanoseconds, with minimal fluctuations, indicating robust ligand-receptor interactions. Pharmacokinetic evaluations based on Lipinski's rule of five suggest that camptothecin adheres to essential drug-likeness parameters, indicating favorable bioavailability. Despite its low solubility, camptothecin's binding stability and pharmacokinetic profile suggest its potential as an effective therapeutic agent for breast cancer, particularly when combined with drug delivery systems that enhance solubility. Further studies are needed to address its solubility issues and optimize its delivery for clinical applications.
A: OpCBR1 can be used to enhance camptothecin production in Ophiorrhiza pumila through two approaches: (1) Knockout of OpCBR1 increases camptothecin biosynthesis by removing its negative regulatory effect on the OpIO gene. (2) Overexpression of OpCBR1 promotes root growth and biomass, which can indirectly increase camptothecin yield through enhanced biomass engineering. These findings suggest that genetic manipulation of OpCBR1 can be a promising strategy for improving camptothecin production.
A: Overexpression of OpCBR1 in O. pumila hairy roots significantly reduces camptothecin biosynthesis by directly repressing the promoter of the OpIO gene, a key gene in the camptothecin biosynthetic pathway. Conversely, knockout of OpCBR1 increases camptothecin production. This indicates that OpCBR1 acts as a negative regulator of camptothecin biosynthesis.
A: OpCBR1 negatively regulates camptothecin biosynthesis by directly repressing the OpIO promoter, reducing camptothecin accumulation. Conversely, it promotes root growth by upregulating auxin and cytokinin-related genes, enhancing biomass. This dual role highlights ERF transcription factors as important regulators of plant metabolism and biomass engineering.