Trifluoroacetic acid
CAS number: 76-05-1
Trifluoroacetic acid is a monocarboxylic acid that is the trifluoro derivative of acetic acid. It has a role as a reagent and a human xenobiotic metabolite.
Related images
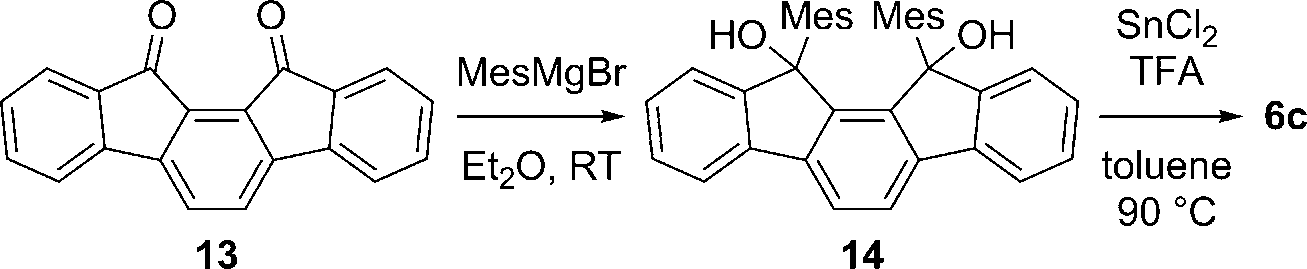
Synthesis of 6c. Mes: 2,4,6-trimethylphenyl, TFA: trifluoroacetic acid.
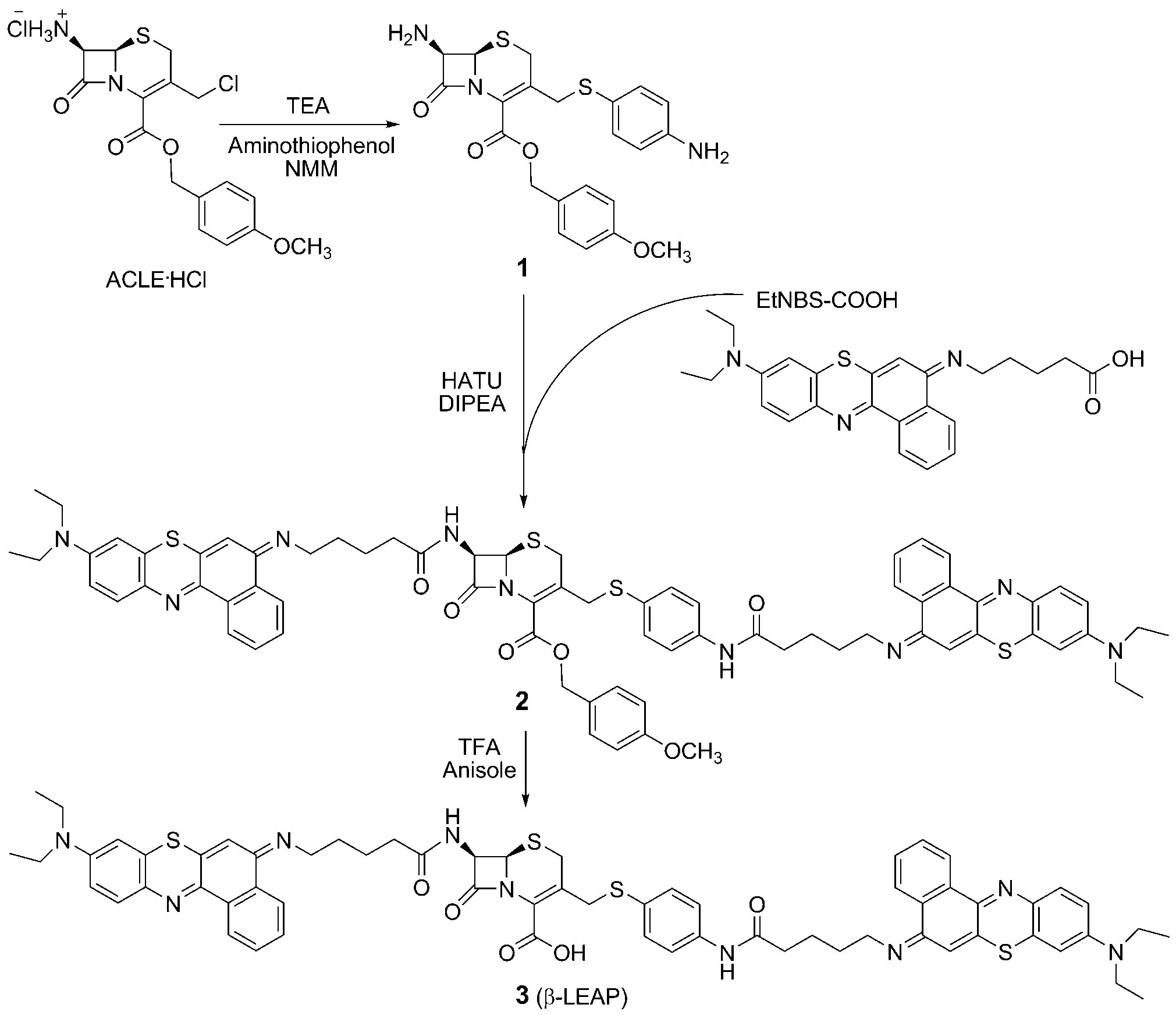
Synthesis of β-LEAP. DIPEA=N,N’-diisopropylethylamine, NMM=4-methylmorpholine, HATU=O-(7-azabenzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate, TEA=triethylamine, TFA=trifluoroacetic acid.
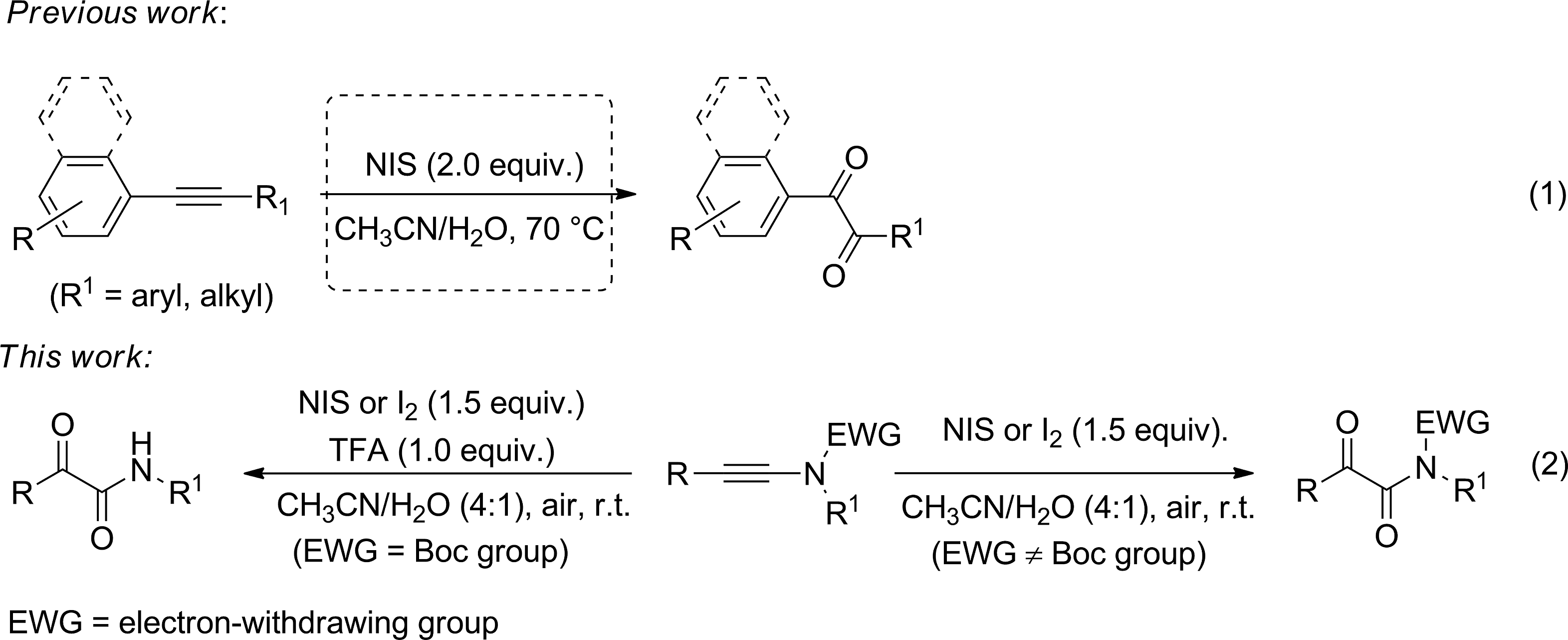
Iodine-mediated oxidation of ynamides (Boc = tert-butoxycarbonyl, TFA = trifluoroacetic acid).
Related Questions and Answers
A: The study highlights that TFA concentrations in Antarctic ice cores have been increasing steadily, indicating ongoing accumulation in environmental sinks. This suggests that anthropogenic sources, particularly the atmospheric degradation of HFCs and u-HFCs, are contributing to the rising levels of TFA. The environmental pathways for the breakdown and removal of TFA are few and poorly understood, indicating a need for further research to mitigate its long-term environmental impact.
A: The study presents the first seasonal record of TFA in Antarctic ice cores, revealing that TFA concentrations increased by 5.8% per year from 2011 to 2021. TFA peaks were observed in September–November, suggesting that polar vortex weakening affects deposition. The analysis was performed using a new non-suppressed ion chromatography-tandem mass spectrometry (nsIC-MS/MS) method, which offers low detection limits, excellent repeatability, and high sensitivity. The method allows for direct injection of ice core samples, eliminating the need for extensive sample preconcentration or complex derivatization steps.
A: Trifluoroacetic acid (TFA) is used to dehydrate compound 12, resulting in the formation of the enone intermediate (13). This step is necessary to introduce the required double bond in the molecule, facilitating further transformations in the synthesis.
A: TFA is used to deprotect the hydroxyl group in the benzyl alcohol intermediates (2a and 2b), while TES acts as a source of hydride ions to reduce the carbonyl group, resulting in the formation of the benzyl derivatives 3a and 3b in high purity and yield (98% for both compounds).