Dimethylamine
CAS number: 124-40-3
Dimethylamine, aqueous solution appears as a solution of a gas in water. Odor ranging from a fishlike to ammonia-like depending on vapor concentration. Corrosive to skin and eyes. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion.
Related images
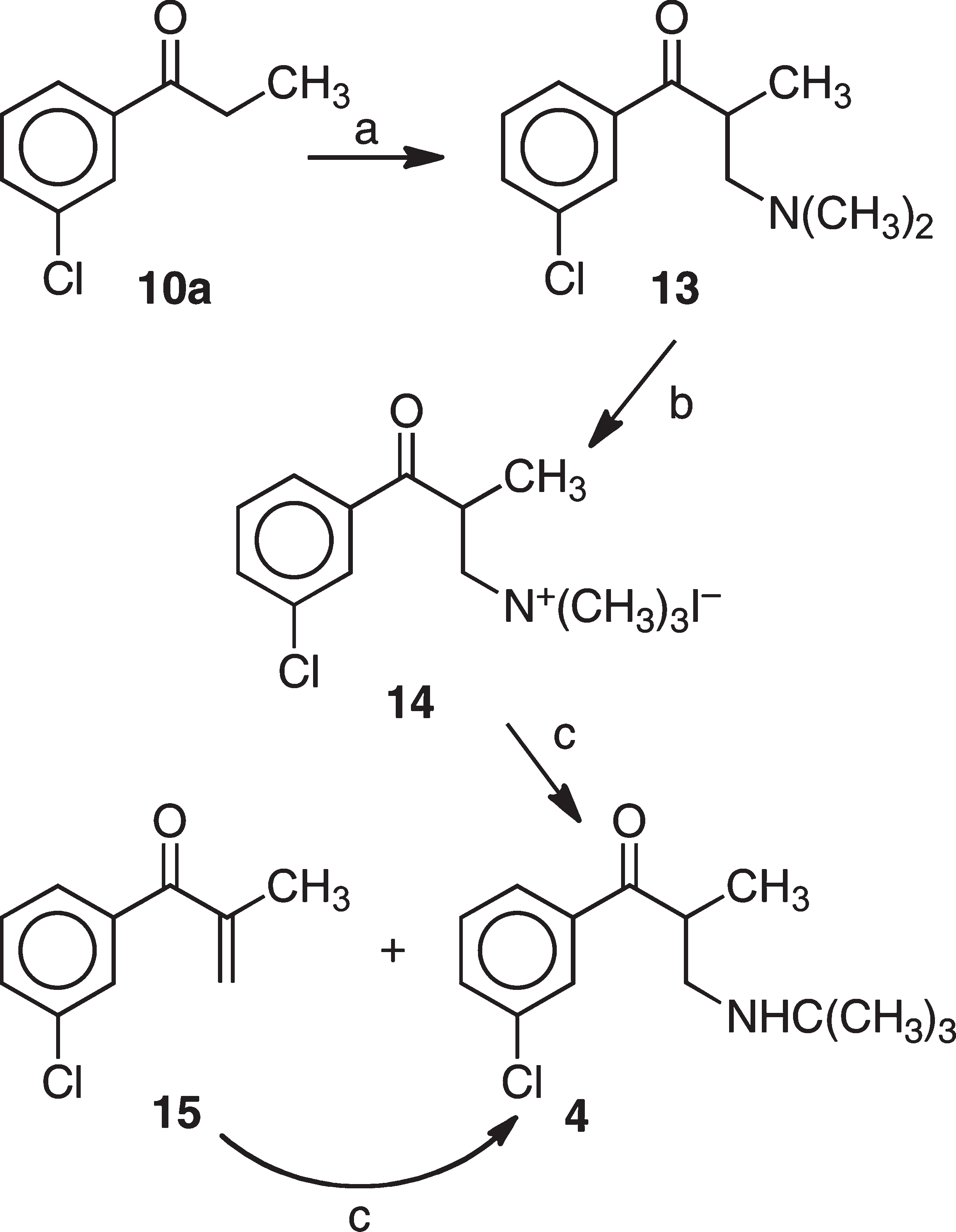
Scheme 3 shows the procedure used for the synthesis of 4. Subjection of 3'-chloropropiophenone (10a) to Mannich reaction conditions with aqueous formaldehyde and dimethylamine gave 13. The methiodide 14 was obtained by alkylation of 13 with iodomethane. Treatment of 14 with tert-butylamine gave first a mixture of 15 and the desired 4. Subjection of the mixture to excess tert-butylamine provided the desired target compound.
Related Questions and Answers
A: DMA has a lower steric hindrance (buried volume of 40.2%) compared to DEA (buried volume of 51.4%), making it easier for DMA to coordinate with TEAL and adsorb onto the catalyst surface. Additionally, DMA has a slightly higher electrophilicity index (20.06 kcal/mol) compared to DEA (19.87 kcal/mol), indicating that DMA can more readily accept electrons and form stronger complexes.
A: The adsorption energy of TEAL·DMA on the Ziegler–Natta catalyst surface is −22.9 kcal/mol in the gas phase and −25.4 kcal/mol in n-hexane, while for TEAL·DEA, the energies are −18.2 kcal/mol and −14.9 kcal/mol, respectively. This shows that TEAL·DMA binds more strongly to the catalyst surface, especially in the non-polar solvent n-hexane, leading to more effective deactivation.
A: The kinetic barrier for the formation of the TEAL·DMA complex is 27.11 kcal/mol, while the barrier for TEAL·DEA complex formation is much higher at 126.05 kcal/mol. This difference in activation energy explains why DMA can more easily deactivate the catalyst compared to DEA.
A: Dimethylamine (DMA) and diethylamine (DEA) both deactivate Ziegler–Natta catalysts by forming complexes with triethylaluminum (TEAL), which then adsorb onto the catalyst surface, blocking active sites. DMA forms a more stable complex with TEAL and adsorbs more strongly onto the catalyst surface, leading to significant deactivation even at lower concentrations.