Sodium Methoxide
CAS number: 124-41-4
Sodium methylate is a white amorphous powder. It reacts with water to form sodium hydroxide, a corrosive material, and methyl alcohol, a flammable liquid. The heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. It is used to process edible fats and oils, and to make other chemicals.
Related images
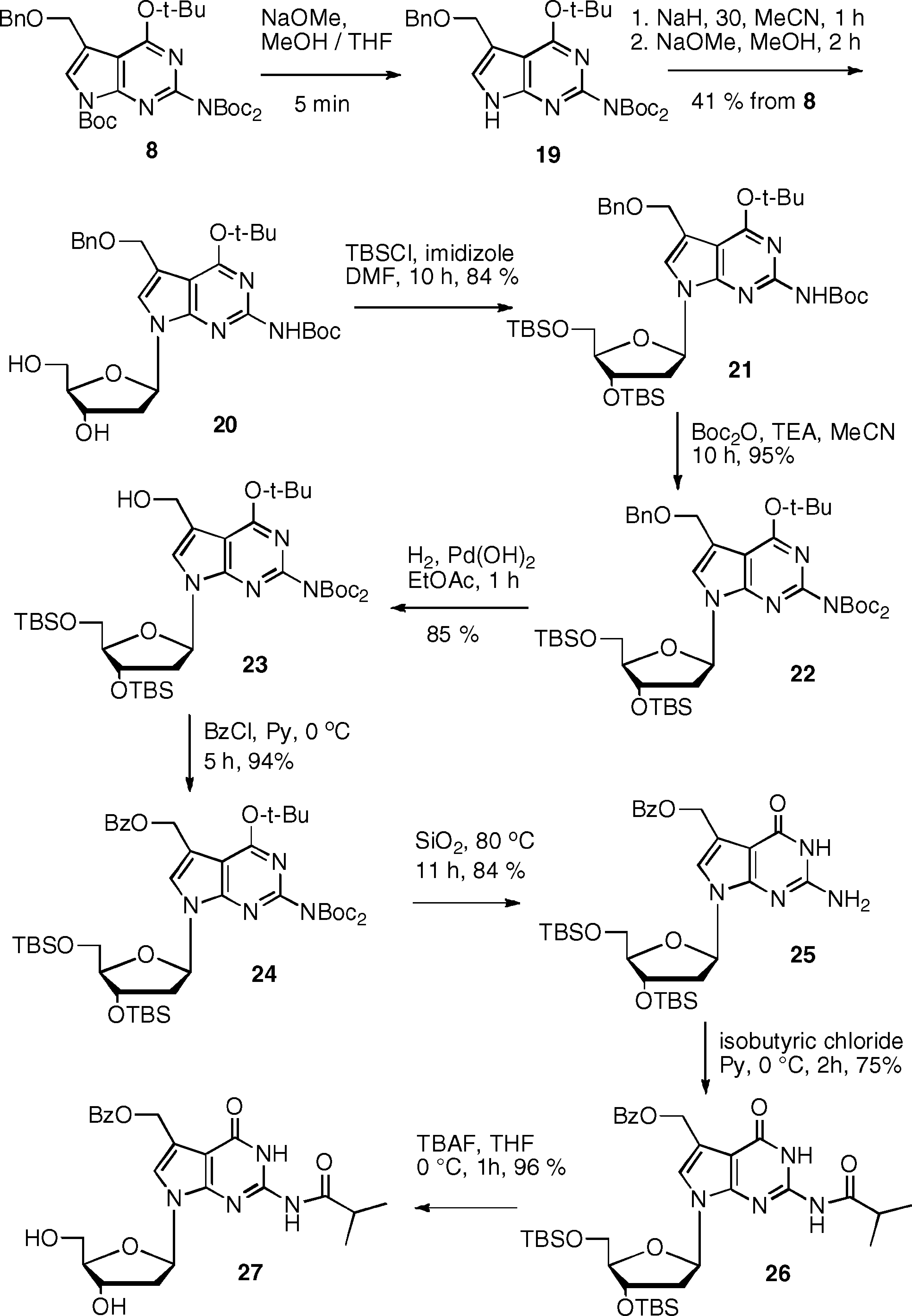
The conversion of 8 to the deoxynucleoside 27 involved its coupling to the chlorosugar after selective removal of the N7-Boc group with NaOMe (Scheme 4).
Related Questions and Answers
No related questions yet