Rivastigmine
CAS number: 123441-03-2
Rivastigmine is a parasympathomimetic or cholinergic agent for the treatment of mild to moderate dementia of the Alzheimer's type. Rivastigmine is a cholinesterase inhibitor that inhibits both butyrylcholinesterase and acetylcholinesterase.
Related images
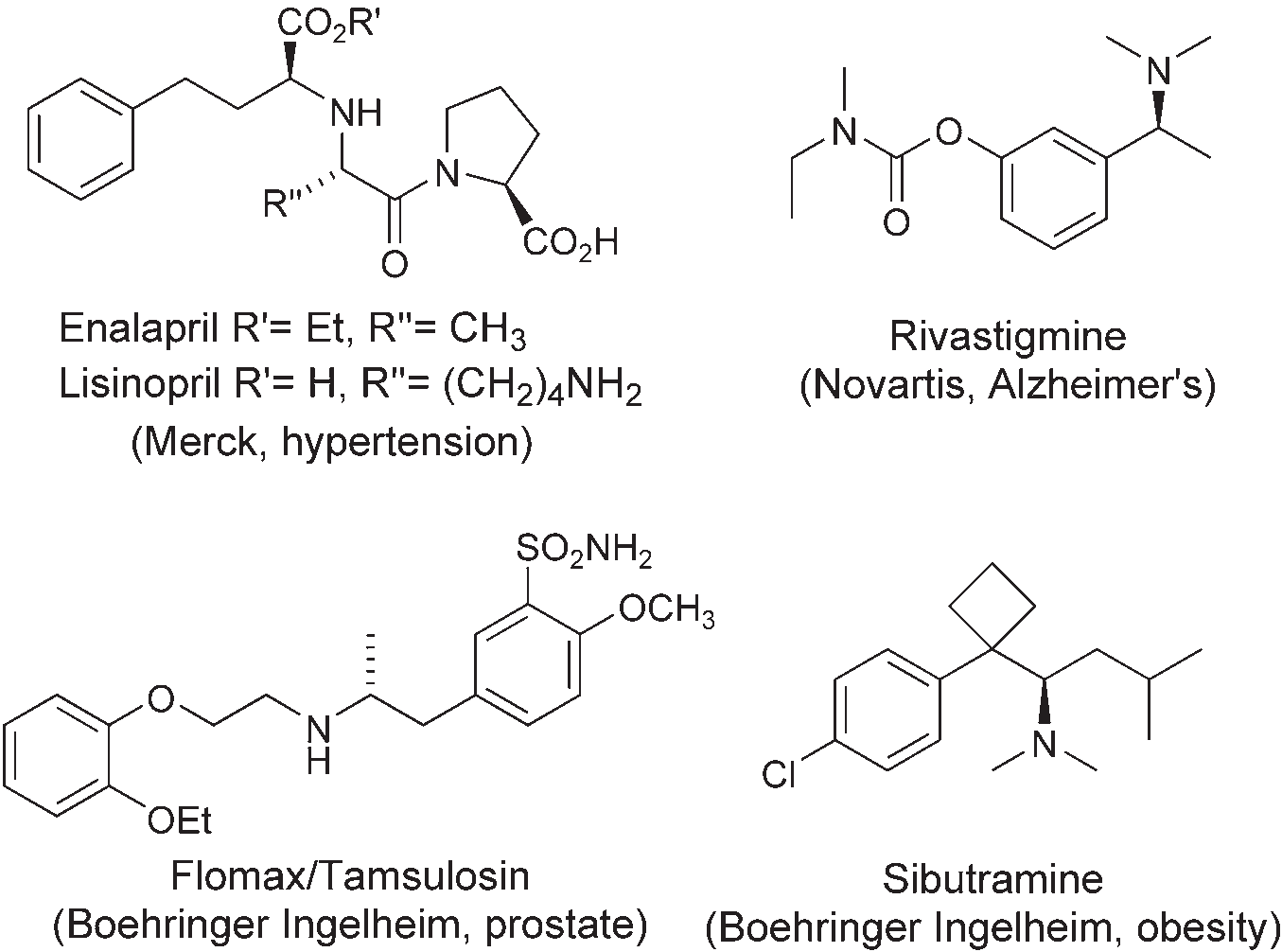
Pharmaceutical drugs containing a-chiral amine stereocenters: enalapril, lisinopril, rivastigmine, Flomax, TAMSULOSIN, sibutramine.
Related Questions and Answers
A: The rivastigmine hybrids were tested for their protective effects against oxidative stress induced by iron/ascorbate and Aβ1–42. Compounds 4AY2, 4AY4, and 4BY2 showed significant neuroprotective effects against oxidative stress, restoring cell viability to levels close to untreated controls. However, none of the tested compounds were able to prevent cytotoxicity induced by Aβ1–42. These findings highlight the potential of these hybrids to modulate mitochondrial or redox homeostasis, although further studies are needed to elucidate their mechanisms of action.
A: The rivastigmine hybrids generally exhibited higher polar surface area (PSA) and lipophilicity (clog Po/w) compared to rivastigmine. These properties are important for drug permeability and bioavailability. The predicted log BB values for the hybrids were lower than those for rivastigmine, indicating potentially reduced BBB permeability. However, the Caco-2 and MDCK permeability values varied, with some hybrids showing higher permeability than rivastigmine. None of the compounds violated Lipinski's Rule of Five, suggesting they have good potential for oral bioavailability.
A: Molecular docking revealed that most rivastigmine hybrids adopted a binding orientation comparable to the most potent inhibitors (4AY4 and 4AY6) within the active site of hAChE. Key interactions included contacts with residues such as TRP86, TYR337, and HIS447. For hBChE, the most potent inhibitor (4BY3) exhibited dual anchoring interactions, with both the acid-derived moiety and rivastigmine fragment positioned close to the catalytic site. Structural modifications, such as the addition of an alkene group, influenced the orientation and accommodation of the hybrids within the enzyme binding site, potentially enhancing key interactions.
A: All rivastigmine hybrids demonstrated significant Aβ1–42 aggregation inhibitory activity, with values ranging from 45% to 82%. The catechol-containing hybrids (4AY5 and 4AY6) showed the highest inhibitory activity, comparable to curcumin (77% inhibition). The presence of free phenolic hydroxyl groups, as in catechol derivatives, contributed to higher inhibitory activity. Structural modifications, such as adding an extra alkene group, also enhanced Aβ aggregation inhibition.
A: The rivastigmine hybrids incorporating Trolox (4AY2 and 4BY2) and catechol groups (4AY5 and 4AY6) exhibited high antioxidant activity, with EC50 values ranging from 15.7 to 28 μM. These values are comparable to the reference compound Trolox (EC50 = 13.8 μM). The syringic acid derivatives (4AY1, 4BY1, and 4CY1) and piperic acid derivatives (4AY3 and 4BY3) showed lower antioxidant activity, likely due to the absence of free phenolic hydroxyl groups.
A: A study designed and synthesized a series of rivastigmine hybrids incorporating antioxidant motifs. These hybrids demonstrated significant antioxidant activity, inhibitory effects on cholinesterases (AChE and BChE), and the ability to inhibit Aβ1–42 aggregation. The best multifunctional capacity was observed in the caffeic acid derivatives (4AY5 and 4AY6), while the Trolox derivatives (4AY2 and 4BY2) showed the best neuroprotective activity against oxidative damage. Molecular docking studies provided insights into the binding modes of these hybrids to cholinesterases, highlighting key interactions despite some lack of correlation with inhibitory potency.