Tetrahydrofuran
CAS number: 109-99-9
Tetrahydrofuran appears as a clear colorless liquid with an ethereal odor. Less dense than water. Flash point 6 °F. Vapors are heavier than air.
Related images
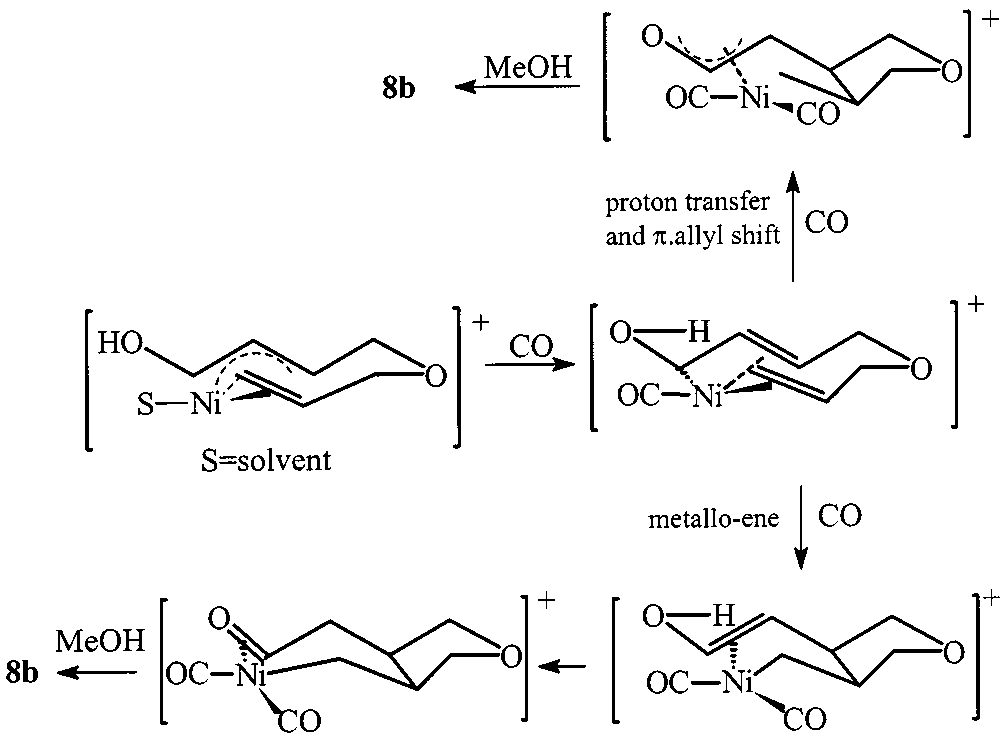
Two plausible paths for the formation of tetrahydrofuran.
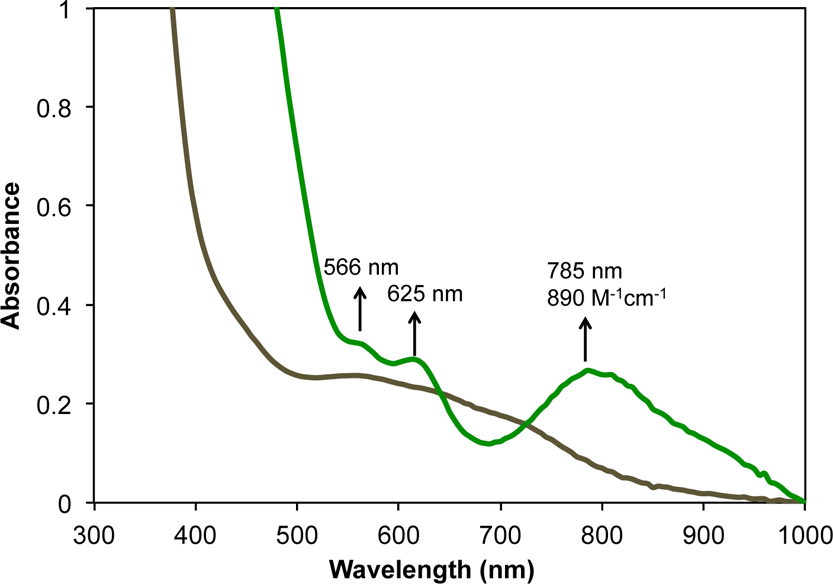
UV-vis spectral change for the conversion of 3 (brown trace) to 4 (green trace) with 0.5% Na/Hg amalgam in tetrahydrofuran (THF).
Related Questions and Answers
A: Tetrahydrofuran (THF) and 2-methyltetrahydrofuran (2-MeTHF) act as both solvents and radical initiators in the reaction. Under violet light irradiation, these solvents generate radical species that initiate the cyclization process, leading to the formation of lactams. The reactions are carried out under air, demonstrating the solvents' ability to support radical formation in the presence of oxygen.
A: A study indicates the significance of the tetrahydropyran ring, as it is essential for presenting the epoxide towards the spliceosome, allowing for covalent binding to the PHF5A-SF3B1 complex. Analogs lacking the epoxide moiety were far less potent due to the absence of covalent binding. The tetrahydrofuran analog 1, despite containing the epoxide, may not position it correctly for covalent bond formation with the reactive cysteine in PHF5A.
A: The tetrahydrofuran analog 1 was found to be three orders of magnitude less potent than meayamycin. While less potent, it still exhibits low micromolar growth inhibition.